Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
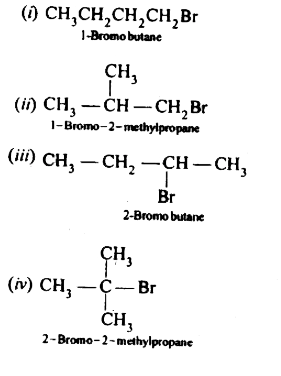
- Write the isomers of the compound having formula C(4)H(9)Br.
Text Solution
|
- The number of optically active compounds in the isomers of C(4)H(9)Br ...
Text Solution
|
- Write the isomers of the compound having formula C(4)H(9)Br .
Text Solution
|
- C(4)H(9)Br सूत्र केवल योगिक के सभी समावयवी लिखिए ।
Text Solution
|
- सूत्र C(4)H(9)Br युक्त यौगिक के समावयवी लिखिए -
Text Solution
|
- C(4)H(9)Br सूत्र वाले यौगिक के सभी समावयवी लिखिए।
Text Solution
|
- Write the isomers of the compound having molecular formula C(4) H(9) B...
Text Solution
|
- Write the isomers of the compound having formula C(4)H(9)Br .
Text Solution
|
- C(4)H(9)Br सूत्र वाले यौगिक के सभी समावयवी लिखिए।
Text Solution
|