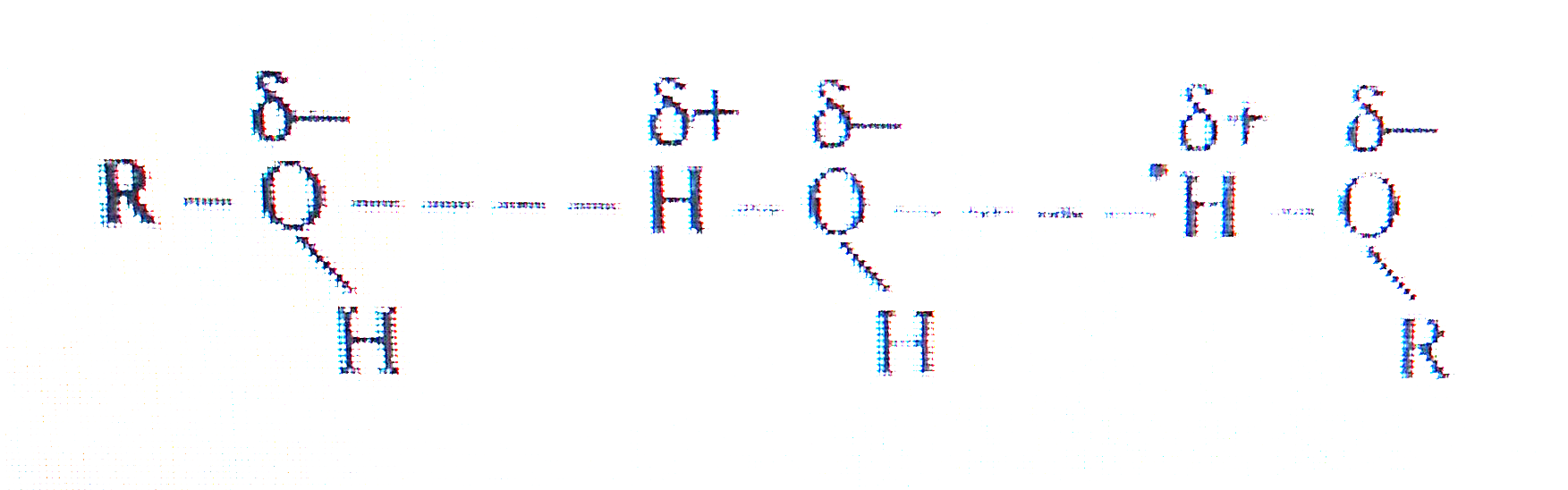Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- समतुल्य आणविक भार वाले हाइड्रोकार्बन की अपेक्षा ऐल्कोहॉल जल में अधिक व...
Text Solution
|
- समतुल्य आण्विक भार वाले हाइड्रोकार्बनों की अपेक्षा ऐल्कोहॉल जल में अधि...
Text Solution
|
- ऐल्कोहॉल समतुल्य अणुभार युक्त हाइड्रोकार्बनों की अपेक्षा जल में अधिक व...
Text Solution
|
- Alcohols are compartively more soluble in water than hydrocarbons of c...
Text Solution
|
- ऐल्कोहॉल संगत हाइड्रोकार्बन की तुलना में पानी में ज्यादा घुलनशील होते ...
Text Solution
|
- समतुल्य अणुभार वाले हाइड्रोकार्बनों की अपेक्षा ऐल्कोहॉल जल में अधिक वि...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|