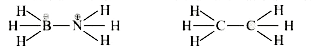A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Choose the correct combination of true statements from the statements ...
Text Solution
|
- The molecule having zero dipole moment is
Text Solution
|
- कथन : BF(3) अणु का परिणामी द्विध्रुव आघूर्ण शून्य होता है । क...
Text Solution
|
- Choose the correct statement regarding SeOCl(2) molecule :
Text Solution
|
- Out of two tetrahedral molecules 'X' and 'Y', molecule 'X' has more di...
Text Solution
|
- Statement I : Bond angle of BF(3) " and " NF(3) are different State...
Text Solution
|
- Choose the correct combination of true statements from the statements ...
Text Solution
|
- The molecule having zero dipole moment is
Text Solution
|
- Choose the correct statement regarding SeOCl(2) molecule :
Text Solution
|