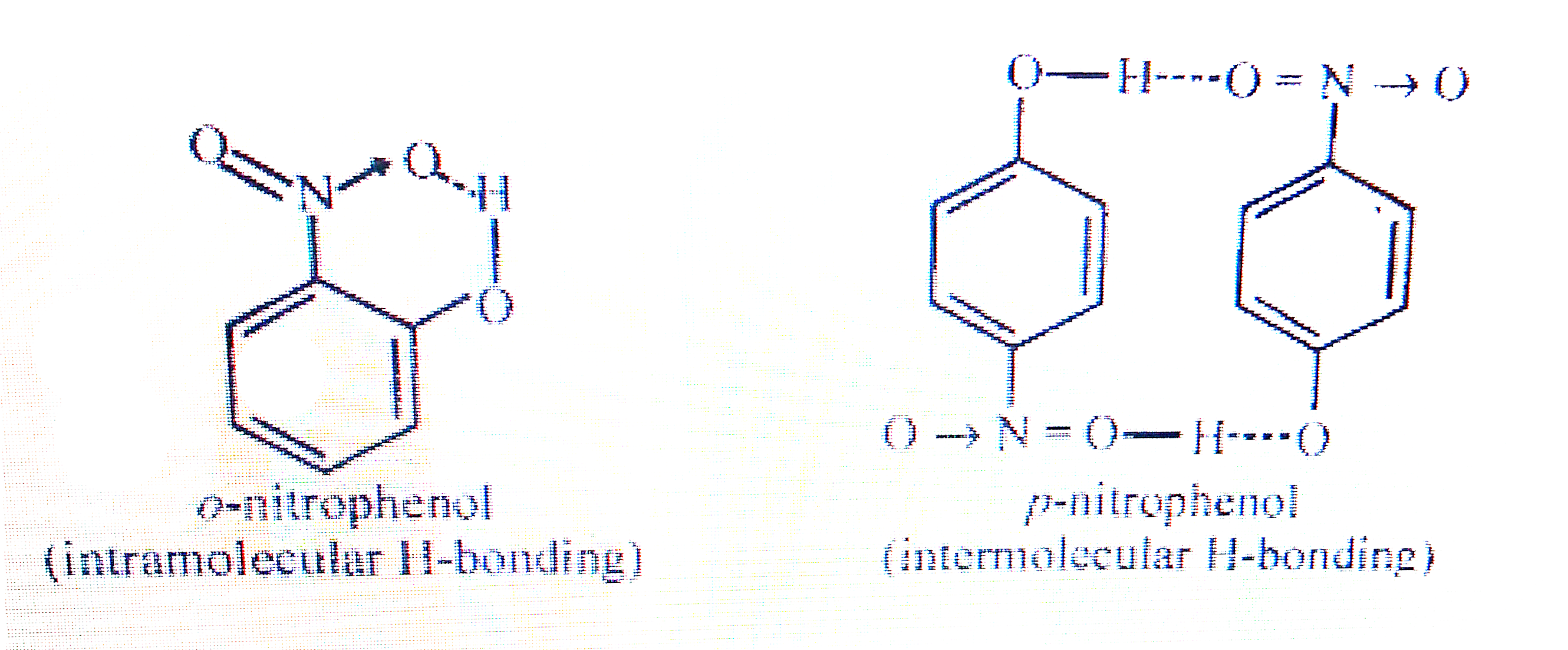Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- While separating a mixture of ortho- and para-nitrophenols steam disti...
Text Solution
|
- While separating a mixture of ortho- and para-nitrophenols steam disti...
Text Solution
|
- Assertion (A) o-and p-nitrophenol can be separated by steam distillati...
Text Solution
|
- ऑर्थो तथा पैरा -नाइट्रोफीनालो के मिश्रण को भाप-आसवन द्वारा पृथक करने म...
Text Solution
|
- Assertion : A mixture of o-nitrophenol and p-nitrophenol can be separa...
Text Solution
|
- ऑर्थो (o-) तथा पैरा (p-) नाइट्रोफिनोलो को भाप आसवन द्वारा अलग-अलग करने...
Text Solution
|
- While separating a mixture of ortho and para nitrophenols by steam dis...
Text Solution
|
- ऑर्थों तथा पैरा-नाइट्रोफीनॉलों के मिश्रण को भाप आसवन द्वारा पृथक करने ...
Text Solution
|
- Ortho isomer of nitrophenol is steam volatile due to .
Text Solution
|