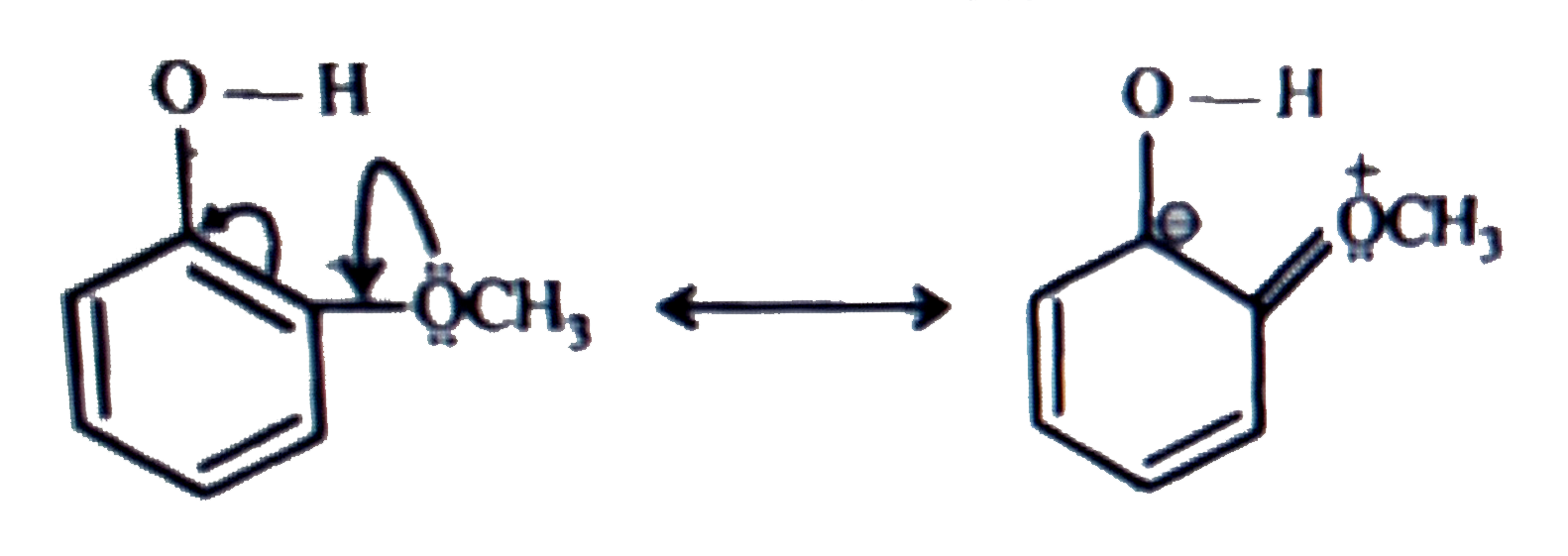Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
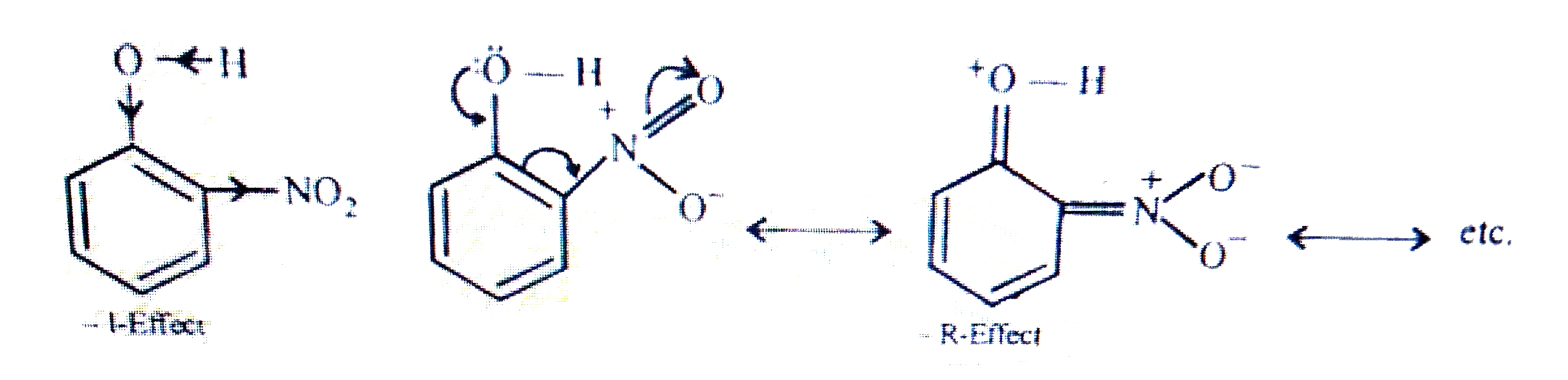
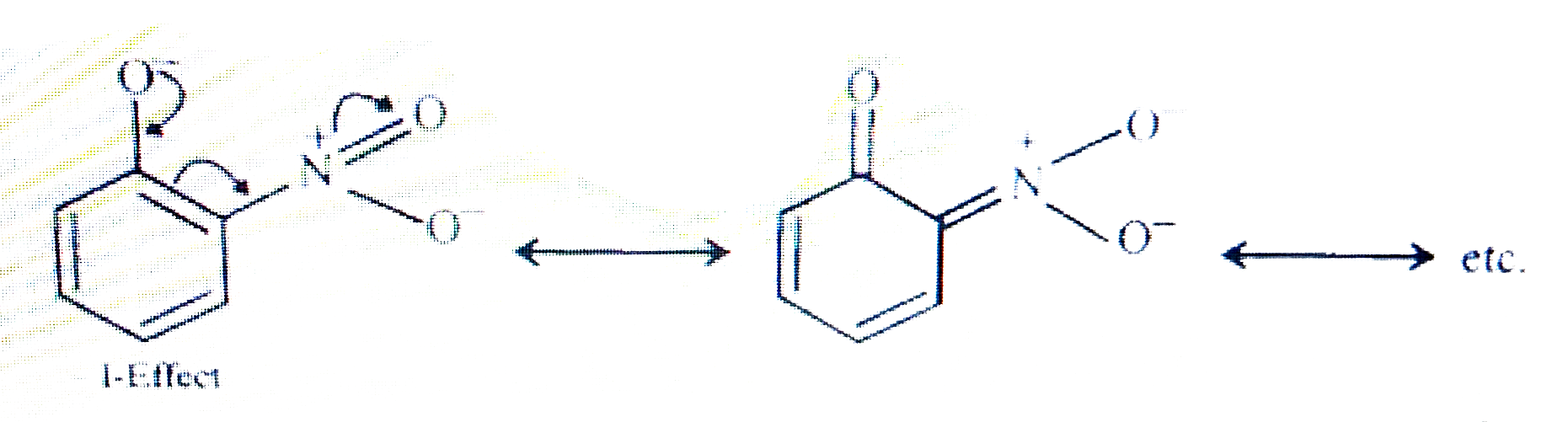
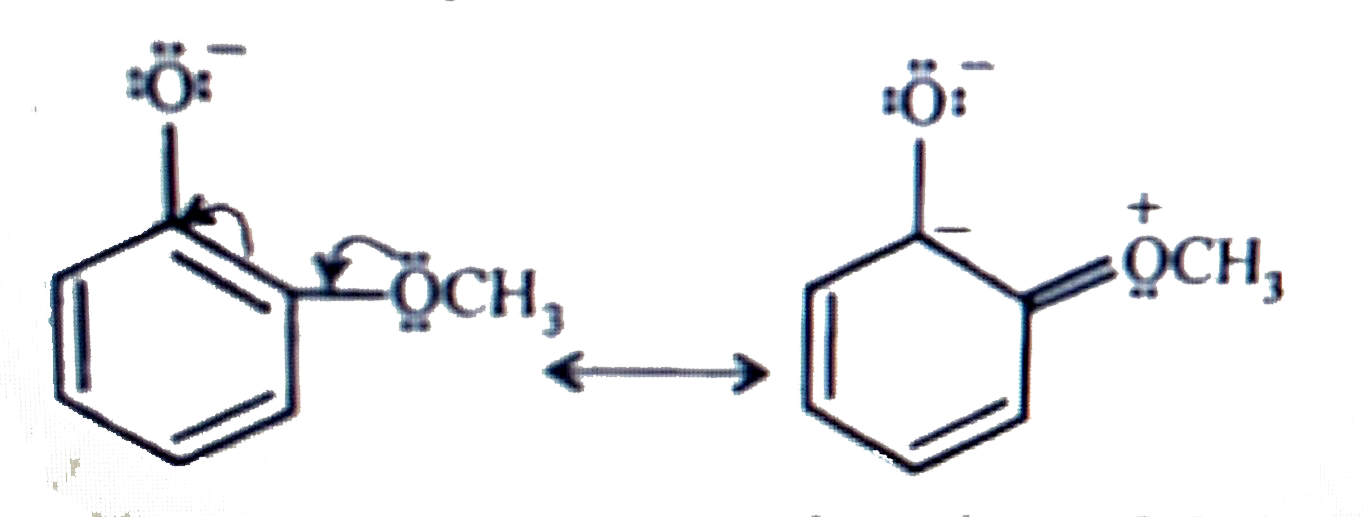
- Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol ...
Text Solution
|
- Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol ...
Text Solution
|
- समझाइए की क्यों ऑर्थोनाइट्रो फीनॉल, ऑर्थो मेथॉक्सी फीनॉल से अधिक अम्ल...
Text Solution
|
- o-नाइट्रोफिनॉल o-मेथोक्सीफिनॉल की तुलना में अधिक अम्लीय क्यों है? स्पष...
Text Solution
|
- Explain Ortho nitrophenol is more acidic than Ortho methoxyphenol.
Text Solution
|
- समझाइये क्यों आर्थों-नाइट्रोफिनॉल आर्थों-मिथाक्सी-फिनॉल से ज्यादा अम्ल...
Text Solution
|
- O - मैथक्सीफिनॉल की अपेक्षा O - नाइट्रोफीनॉल अधिक अम्लीय है समझाइ...
Text Solution
|
- समझाइए कि क्यों ऑर्थों नाइट्रोफीनॉल, ऑर्थों मेथॉक्सीफीनॉल से अधिक अम्ल...
Text Solution
|
- (a) (i) Explain why is ortho nitrophenol more acidic than ortho methox...
Text Solution
|