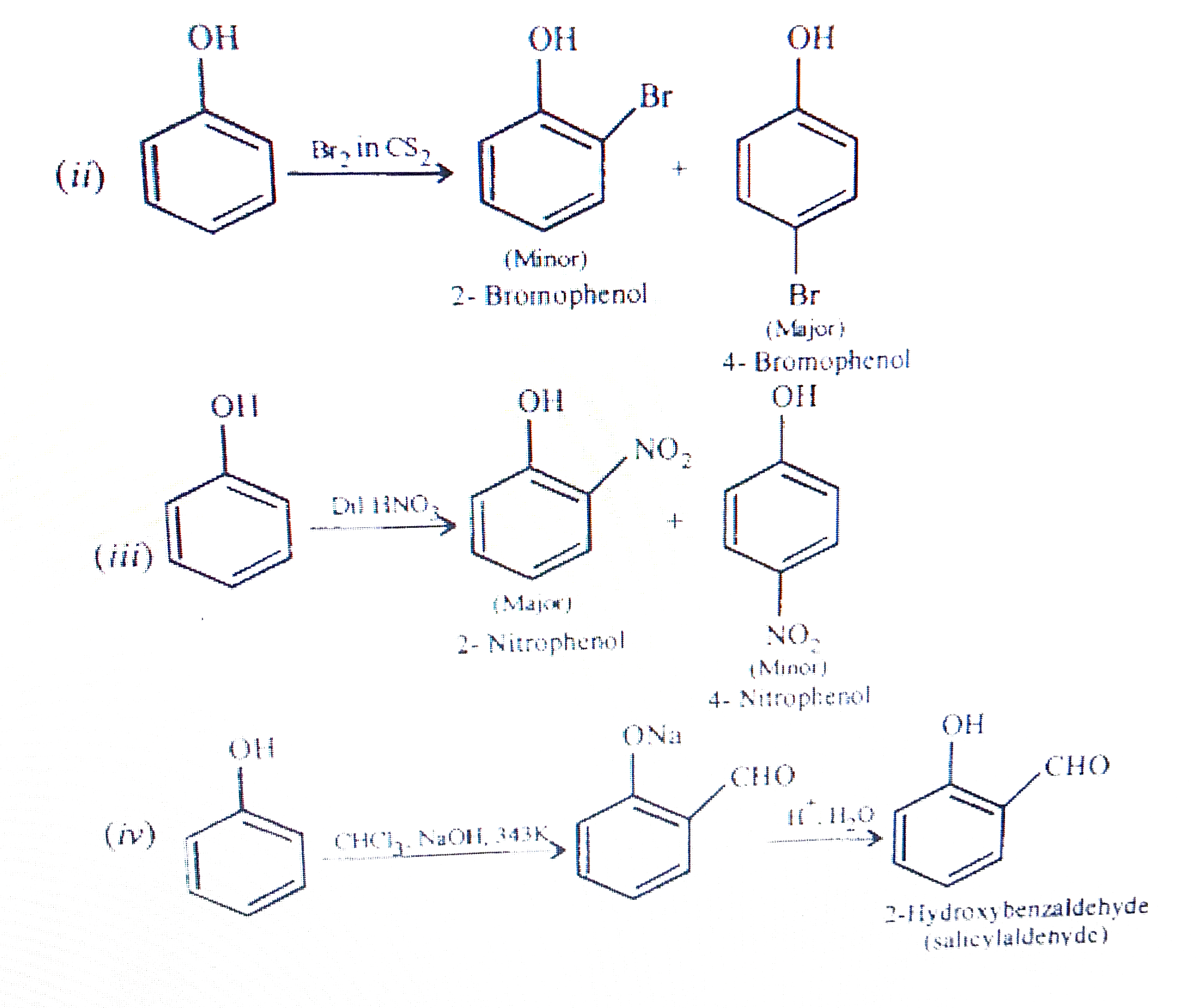
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Give the equations of the following reactions: i. Oxidation of prop...
Text Solution
|
- Give the equations of the following reactions: i. Oxidation of prop...
Text Solution
|
- Give equations of the following reactions: (i) Reaction or propene w...
Text Solution
|
- प्रोपेन -1-आल का क्षारीय KMnO(4) के साथ ऑक्सीकरण (ii) ब्रोमिन की CS(2)...
Text Solution
|
- निम्न अभिक्रिया के लिए समीकरण दीजिए: फीनॉल की जलीय NaOH की उपस्थिति ...
Text Solution
|
- निम्न अभिक्रियाओं की समीकरण लिखिये- (i) क्षारीय KMnO(4) विलयन द्वारा...
Text Solution
|
- The reaction between phenol and chloroform in the presence of aqueous ...
Text Solution
|
- फिनॉल कि क्लोरोफॉर्म के साथ जलीय NaOH की उपस्थिति में क्रिया | अभिक्रि...
Text Solution
|
- निम्न अभिक्रियाओं के समीकरणों को लिखिए - जलीय NaOH की उपस्थिति...
Text Solution
|