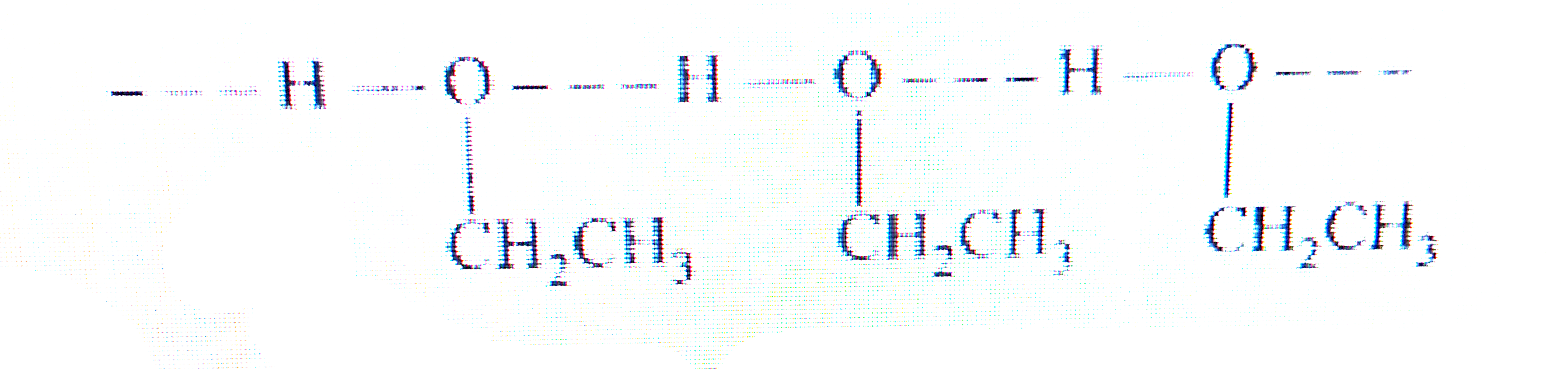Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Give reason for the higher boiling point of ethanol in comparison to m...
Text Solution
|
- Given reason for the higher boiling point of ethanol in comparison to ...
Text Solution
|
- मेथोक्सीमेथेन की तुलना में एथेनोल के अधिक क्वथनांक का कारण स्पष्ट कीजि...
Text Solution
|
- एथेनॉल का क्वथनांक मिथाक्सीमेथेन की तुलना में उच्च होने का कारण दीजिये...
Text Solution
|
- Give reason for the higher boiling point of ethanol in comparison to m...
Text Solution
|
- डाइमेथिल ईथर की अपेक्षा एथेनॉल का क्वथनांक उच्चतर होता है कारण दीजिए-
Text Solution
|
- Ethanol has higher boiling point than methoxy methane. Give reason.
Text Solution
|
- 2 Give reason for the higher boiling point of ethanol in comparison to...
Text Solution
|
- Give reason for the higher boiling point of ethanol in comparison to m...
Text Solution
|