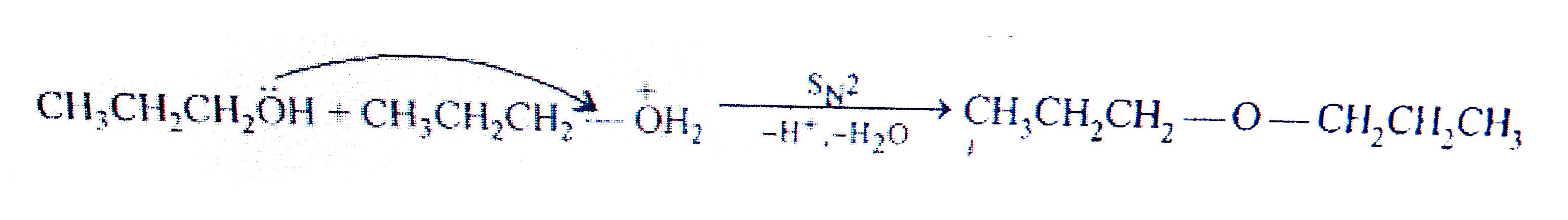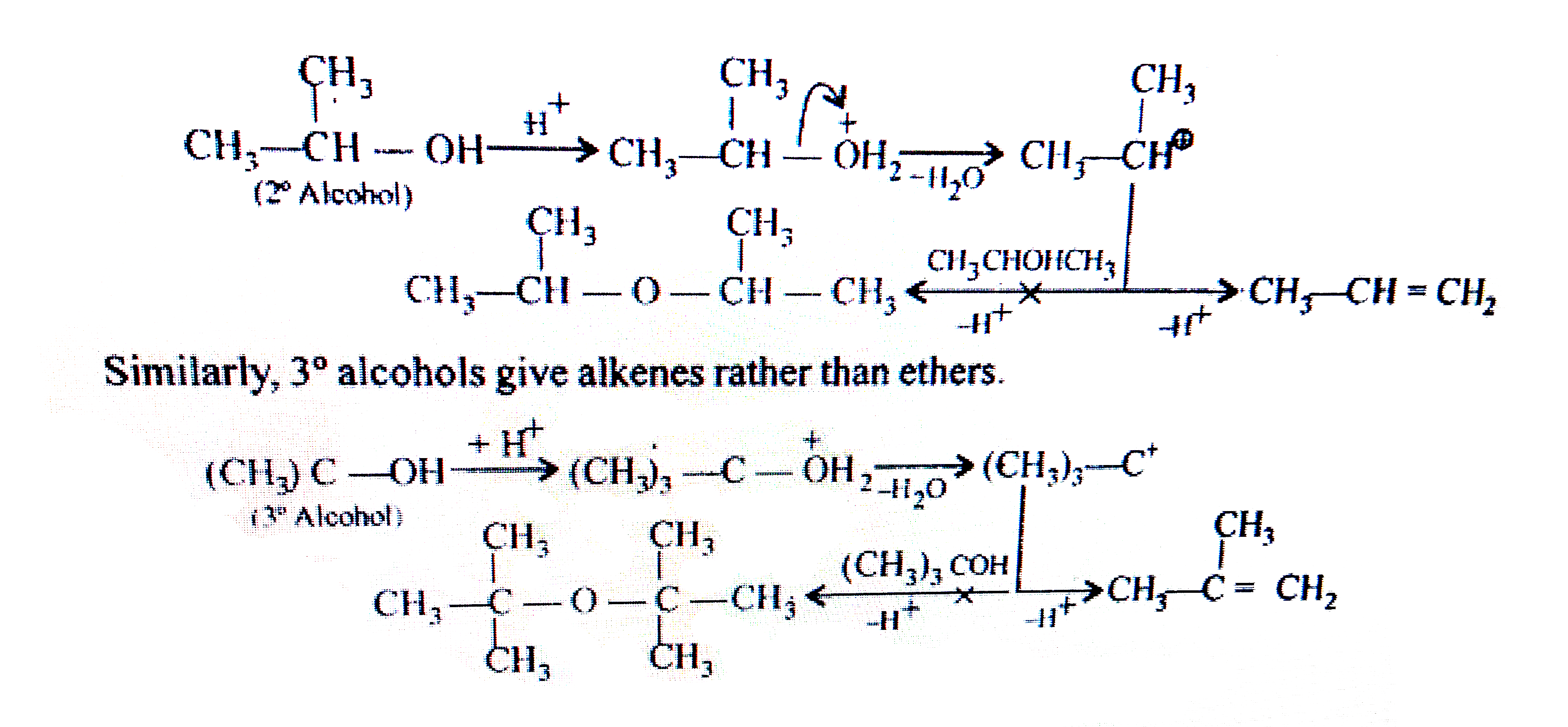Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Preparation of ethers by acid dehydration of secondary or tertiary alc...
Text Solution
|
- Preparation of ethers by acid dehydration of secondary or tetiary alco...
Text Solution
|
- द्वितीयक अथवा तृतीयक ऐल्कोहॉलो के अम्लीय निर्जलन द्वारा ईथरों को बंनान...
Text Solution
|
- द्वितीयक अथवा तृतीयक ऐल्कोहॉलों के आम्लीय निर्जलन द्वारा ईथरों को बनान...
Text Solution
|
- द्वितीयक अथवा तृतीयक ऐल्कोहॉलों का अम्लीय निर्जलीकरण ईथरों के निर्माण ...
Text Solution
|
- Preparation of ethers by acid dehydration of secondary or tertiary alc...
Text Solution
|
- द्वितीयक व तृतीयक ऐल्कोहॉल के अम्ल-निर्जलीकरण द्वारा ईथर का बनना एक उप...
Text Solution
|
- Preparation of ethers by acid dehydration of secondary or tetiary alco...
Text Solution
|
- Preparation of ethers by intermolecular dehydration of alcohols in the...
Text Solution
|