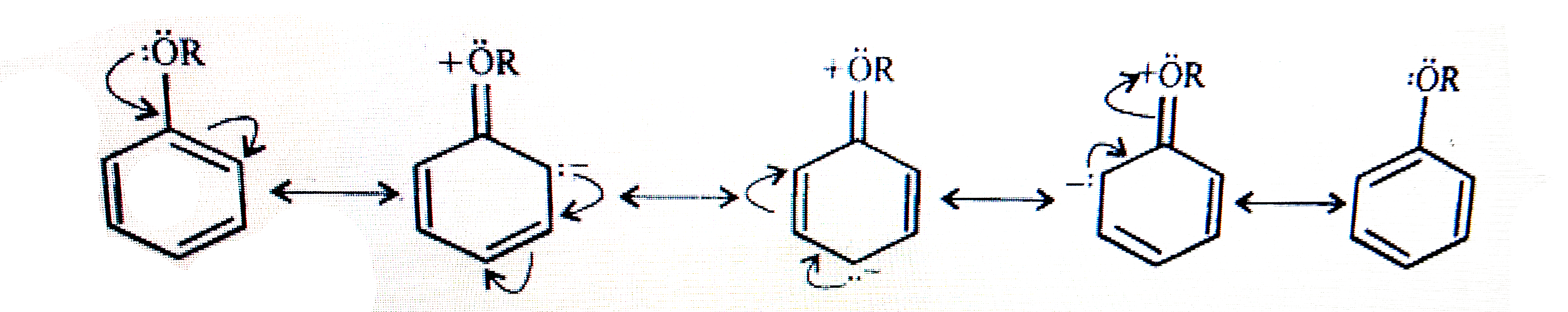Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the fact that in aryl ethers, (i) the alkoxy group activates t...
Text Solution
|
- Explain the fact that in aryl ethers, (i) the alkoxy group activates t...
Text Solution
|
- Explain the fact that in alkyl aryl ethers, alkoxy group : (i) activ...
Text Solution
|
- एरिल ऐल्किल ईथरो में निम्न तथ्यों की व्याख्या कीजिए: (i) ऐल्कॉक्सी स...
Text Solution
|
- निम्न तथ्यों की व्याख्या कीजिए- (i) ऐल्कॉक्सी समूह बैंजीन रिंग को इल...
Text Solution
|
- Explain the fact that in aryl alkyl ethers the alkoxy group activates ...
Text Solution
|
- इस कथन को समझाइये कि एरिल-एल्किल ईथर में (i) ऐल्कॉक्सी समूह बेंजीन रिं...
Text Solution
|
- ऐरिल ऐल्किल ईथरों में निम्न तथ्य की व्याख्या कीजिए - ऐल्कॉक्सी समूह...
Text Solution
|
- Explain the fact that in aryl alkyl ethers (i) the alkoxy group activa...
Text Solution
|