Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-Exercise
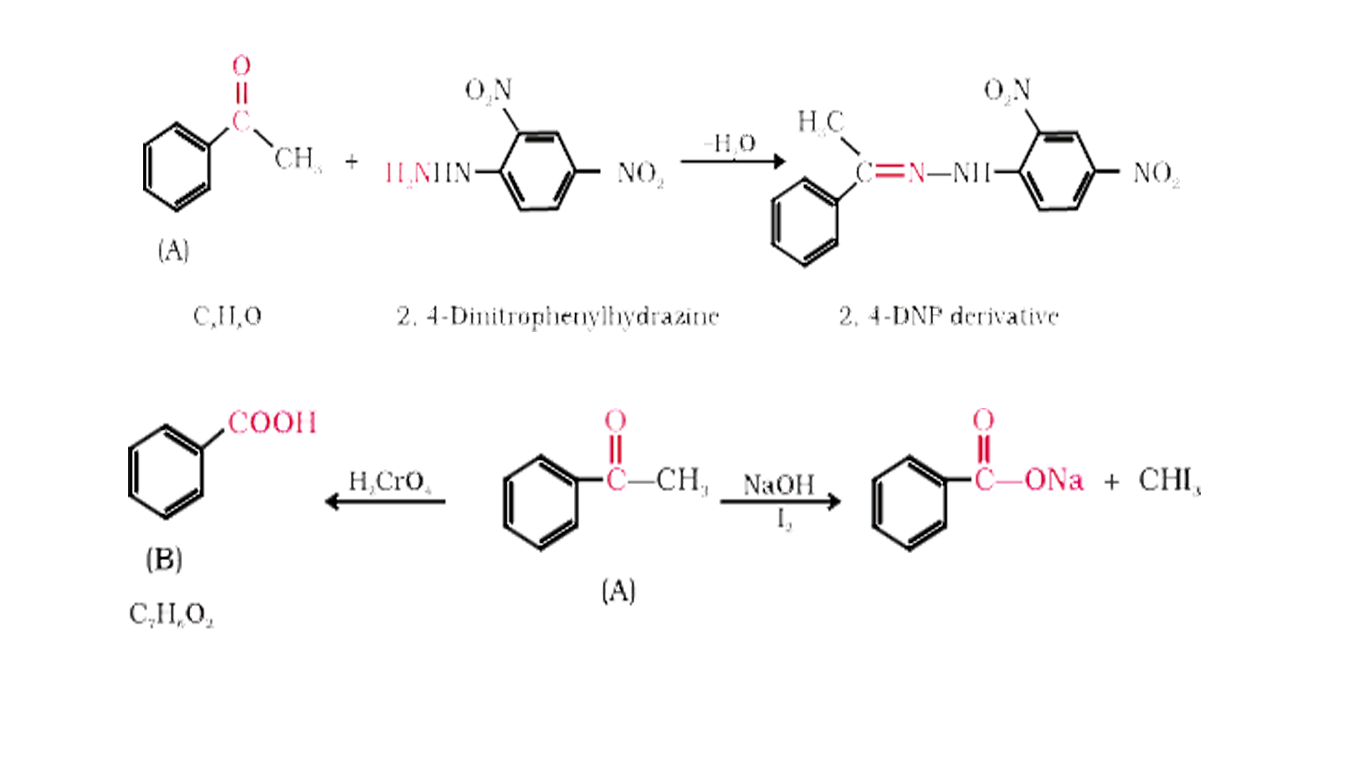
- An organic compound containing (x) with molecular formula C(8)H(8)O fo...
Text Solution
|
- Write the structures of the following compounds. (i) alpha-Methoxypr...
Text Solution
|
- Write the structures of products of the following reactions,
Text Solution
|
- Arrange the following compounds in increasing order of their boiling p...
Text Solution
|
- Arrange the following compounds in increasing order of their reactivit...
Text Solution
|
- Predict the products of the following reactions:
Text Solution
|
- Give the IUPAC names of the following compounds: (i) "Ph CH"(2)CH(2)...
Text Solution
|
- Show how each of the following compounds can be converted to benzoic a...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|
- What is meant by the following terms ? Give an example of the reaction...
Text Solution
|
- Name the following compounds according to the IUPAC system of nomencla...
Text Solution
|
- Draw the structures of following compound: i. 3-Methylbutanal ii. ...
Text Solution
|
- Write the IUPAC names of following ketones and aldehydes. Wherever pos...
Text Solution
|
- Draw the structure of following derivatives: i. 2,4-Dinitrophenylhyd...
Text Solution
|
- Predict the products formed when cyclohexane carbaldehyde reacts with ...
Text Solution
|
- Which of the following compounds would undergo aldol condensation or t...
Text Solution
|
- How will you convert ethanal into the following compounds ? i. Butan...
Text Solution
|
- Write structure formulae and names of four possible aldol condensation...
Text Solution
|
- An organic compound with the molecular folmula C(9)H(10)O form 2,4-DNP...
Text Solution
|
- An organic compound (A) (molecular formula C(8)H(16)O(2)) was hydrolys...
Text Solution
|
- Arrange the following compounds in the increasing order of their prope...
Text Solution
|