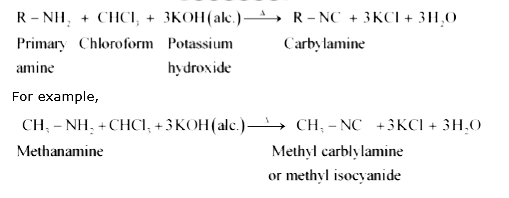
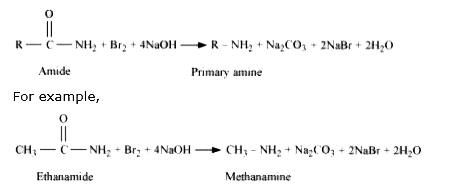
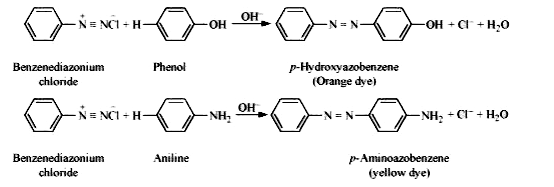
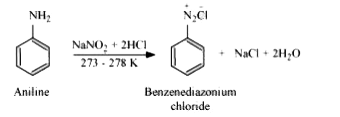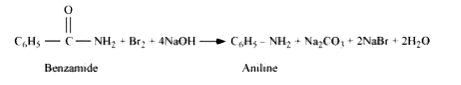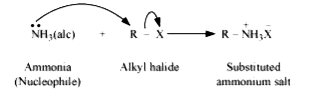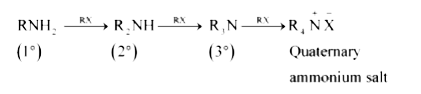Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-AMINES-Exercise
- Arrange the following in increasing order of their basic strength: (...
Text Solution
|
- Complete the following acid-base reactions and name the products: (i...
Text Solution
|
- Write reactions of the final alkylation product of aniline with excess...
Text Solution
|
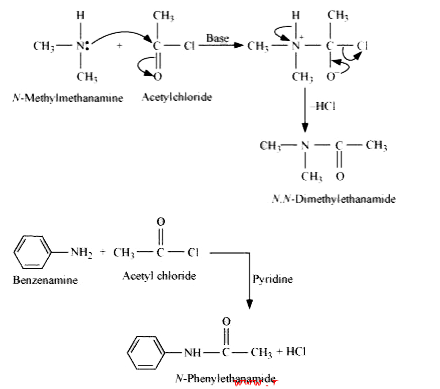
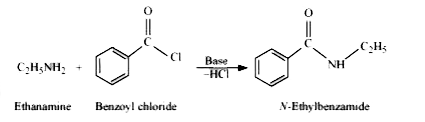
- Write chemical reaction of aniline with benzoyl chloride and write the...
Text Solution
|
- Write structures of different isomers corresponding to the molecular f...
Text Solution
|
- Convert (i) 3-Methylaniline into 3-nitrotoluene. (ii) Aniline into...
Text Solution
|
- Write IUPAC names of the following compounds and classify them into pr...
Text Solution
|
- Give one chemical test to distinguish between the following pairs of c...
Text Solution
|
- Account for the following: (i) pK(b) of aniline is more than that of...
Text Solution
|
- Arrange the following: (i) In decreasing order of the pK(b) values: ...
Text Solution
|
- Convert : i. Ethanoic acid into methylamine ii. Hexanenitrile int...
Text Solution
|
- Describe a method for the identification of primary , secondary and te...
Text Solution
|
- Write short notes on the following : i. Carbylamine reaction ii....
Text Solution
|
- Accomplish the following conversions : i. Nitrobenzene to benzoic ac...
Text Solution
|
- Give the structures of A, B and C in the following reactions: (i) CH...
Text Solution
|
- An aromatic compound (A) on treatment with aqueous ammonia and heating...
Text Solution
|
- Complete the following reactions: (i) C(6)H(5)NH(2)+CHCl(3)+alc.KOHt...
Text Solution
|
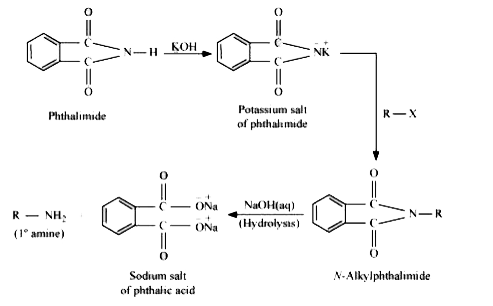
- Why cannot aromatic primary amines be prepared by Gabriel phthalimide ...
Text Solution
|
- Write the reaction of (i) aromatic and (ii) aliphatic primary amines w...
Text Solution
|
- Give explanation for each of the following : (i) Why are amines les...
Text Solution
|