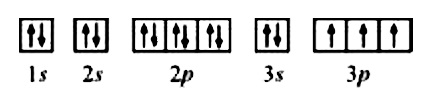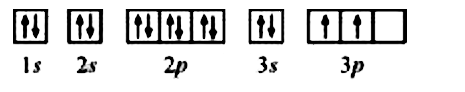Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-STRUCTURE OF ATOM -EXERCISE
- In astronomical observations, signals observed from the distant stars ...
Text Solution
|
- Lifetimes of the molecules in the excited states are often measured by...
Text Solution
|
- The longest wavelength doublet absorption is observed at 589 and 589.6...
Text Solution
|
- The work function for caesium atom is 1.9 eV. Calculate (a) the thresh...
Text Solution
|
- Following results are observed when sodium metal is irradiated with di...
Text Solution
|
- The ejection of the photoelectron from the silver metal in the photoel...
Text Solution
|
- If the photon of the wavelength 150 p m strikes an atom and one of its...
Text Solution
|
- Emission transitions in the Paschen series end at orbit n=3 and start ...
Text Solution
|
- Calculate the wavelength for the emission transition if it starts from...
Text Solution
|
- Dual behaviour of matter proposed by de Broglie led to the discovery o...
Text Solution
|
- Similar to electron diffraction, neutron diffraction microscope is als...
Text Solution
|
- If the velocity of the electron in Bohr's first orbit is 2.19xx10^(6) ...
Text Solution
|
- If the position of the electron is measured within an accuracy of +- 0...
Text Solution
|
- The quantum numbers of six electrons are given below. Arrange them in ...
Text Solution
|
- The bromine atom possesses 3s electrons. It contains six electrons ...
Text Solution
|
- Among the following pairs of orbital which orbital will experience the...
Text Solution
|
- The unpaired electrons in Al and Si are present in 3p orbital. Which e...
Text Solution
|
- Indicate the number of unpaired electrons in: a. P, b. Si, c. Cr, ...
Text Solution
|
- a. How many sub-shell are associated with n = 4? b. How many electr...
Text Solution
|
- a. How many sub-shell are associated with n = 4? b. How many electr...
Text Solution
|