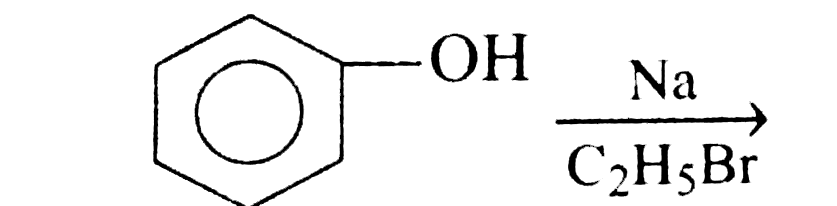
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.50)|25 VideosALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise Linked Comprehension Type|24 VideosALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise Level 2 (Q.126 To Q.132)|7 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-ALCOHOLS AND ETHERS-More Than One Correct (Q.1 To Q.25)
- which of the following ethers will get hydrolysed by HI?
Text Solution
|
- Which of the following reactions are correctly matched?
Text Solution
|
- Which of the following compounds will give positive Victor Meyer test?
Text Solution
|
- Which of the followin alcohols undergo rearrangement during degydratio...
Text Solution
|
- C(2)H(5)0C(2)H(5) and can be distnguished by:
Text Solution
|
- The ether when treated with HI produces:
Text Solution
|
- Which of the following reactions will give ether as main product?
Text Solution
|
- C(2)H(5)Br can be converted into C(2)H(5)-O-C(2)H(5) by:
Text Solution
|
- 1^(@),2^(@)and 3^(@) alcohols can be distinguished by:
Text Solution
|
- Alcohols can be replaced by-Cl group by the followin reagents:
Text Solution
|
- Glycerol can be converted to acrolein by dehydration in presence of
Text Solution
|
- CH(3)CH(2)-OH can be converted to CH(3)CH(2)CN by the following reacti...
Text Solution
|
- Which of the following will oxidise to salt of acid by Br(2)+KOH?
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
- Find the final product A ,B ,C,D of the reaction and choose correct op...
Text Solution
|
Text Solution
|
Text Solution
|