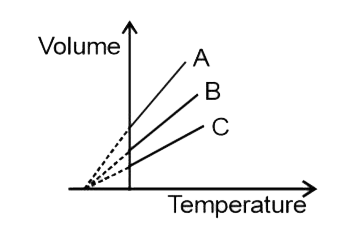
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
KINETIC THEORY OF GASES
MOTION|Exercise EXERCISE-2|37 VideosKINETIC THEORY OF GASES
MOTION|Exercise EXERCISE-3 SECTION-A|23 VideosKINETIC THEORY OF GASES
MOTION|Exercise QUESTION FOR PRACTICE|22 VideosKINEMATICS
MOTION|Exercise EXERCISE 4 PREVIOUS YEAR (LEVEL 2)|6 VideosLOGIC GATES
MOTION|Exercise EXERCISE - 2|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-KINETIC THEORY OF GASES -EXERCISE-1
- An 8 gram sample of a gas occupies 12.3 liters at a pressure of 40.0 c...
Text Solution
|
- A perfect gas at 27^(@)C is heated at constant pressure so as to tripl...
Text Solution
|
- If pressure of a gas contained in a closed vessel is increased by 0.4%...
Text Solution
|
- It is required to double the pressure of helium gas, contained in a st...
Text Solution
|
- The volumes of two vessels are 5 litre and 3 litre respectively. Air i...
Text Solution
|
- The gas equation PV//T = constant is true for a constant mass of an id...
Text Solution
|
- A vessel of volume 4 litres contains a mixture of 8g of O(2), 14g of N...
Text Solution
|
- Two gases each at temperature T, volume V and pressure P are mixed suc...
Text Solution
|
- At a temperature T K, the pressure of 4.0g argon in a bulb is p. The b...
Text Solution
|
- When 2g of gas A is introduced into an evacuated flask kept at 25^(@)C...
Text Solution
|
- A container of volume 30 litre is filled with an ideal gas at one atmo...
Text Solution
|
- Two glass bulbs of equal volume are connected by a narrow tube and are...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is shown in figure. ...
Text Solution
|
- For V versus T curves at constant pressure P1 and P2 for and ideal gas...
Text Solution
|
- Two different masses m and 3 m of an ideal gas are heated separately i...
Text Solution
|
- From the following V-T diagram we can conclude:-
Text Solution
|
- The expansion of an ideal gas of mass m at a constant pressure P is gi...
Text Solution
|
- During an experiment, an ideal gas is found to obey an additional law ...
Text Solution
|
- 12 g of gas occupy a volume of 4xx10^(-3) m^(3) at a temperature of 7...
Text Solution
|
- A container X has volume double that of container Y and both are conne...
Text Solution
|