A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
MATTER WAVE
MOTION|Exercise EXERCISE 3 (SECTION A)|17 VideosMATTER WAVE
MOTION|Exercise EXERCISE 3 (SECTION B)|7 VideosMATTER WAVE
MOTION|Exercise EXERCISE 1 (SECTION B : DAVISSION GERMER EXPERIMENT )|11 VideosMAGNETISM -1
MOTION|Exercise Exercise - 3 SECTION -A|56 VideosMODERN PHYSICS
MOTION|Exercise PHYSICS|73 Videos
MOTION-MATTER WAVE-EXERCISE 2
- According to the modern theory for nature of light, the light has
Text Solution
|
- A proton and an alpha - particle have kinetic energy in the ratio 16:1...
Text Solution
|
- The momentum of photon of frequency 10^9 Hz will be
Text Solution
|
- What is de-Broglie wavelength of an atom of mass m, moving at absolute...
Text Solution
|
- Electron has energy of 100 eV what will be its wavelength &
Text Solution
|
- The ratio of wavelength of deutron and proton accelerated through the ...
Text Solution
|
- The velocity at which the mass of a particle becomes twice of its rest...
Text Solution
|
- The speed of a proton is (c )/(20) . The wavelength associated with it...
Text Solution
|
- If E and P are the energy and the momentum of a photon respectively,th...
Text Solution
|
- The de-Broglie wavelength associated with electrons revolving round th...
Text Solution
|
- From rest an electron is accelerated between two such points which has...
Text Solution
|
- The wavelength of very fast moving electron (v ~~ c) is :
Text Solution
|
- If the mass of neutron is 1.7xx10^(-27) kg, then the de Broglie wavele...
Text Solution
|
- An electron of mass m(e) and a proton of mass m(p) are moving with the...
Text Solution
|
- The magnitude of the de-Broglie wavelength (lambda) of an electron (e)...
Text Solution
|
- The De broglie wavelength associated with protons accelerated through ...
Text Solution
|
- A proton moves on a circular path of radius 6.6 × 10^(-3) m in a perp...
Text Solution
|
- The electron behaves as waves because they can
Text Solution
|
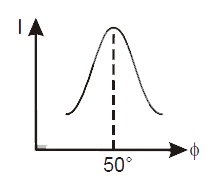
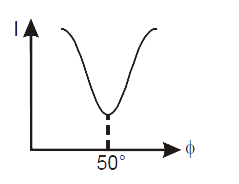
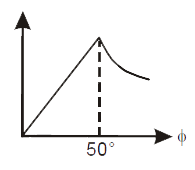
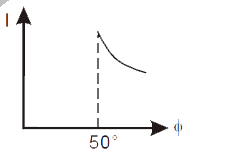
- The correct curve between intensity of scattering (I) and the angle of...
Text Solution
|