Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-CALORIMETRY -EXERCISE - 3 Section - B
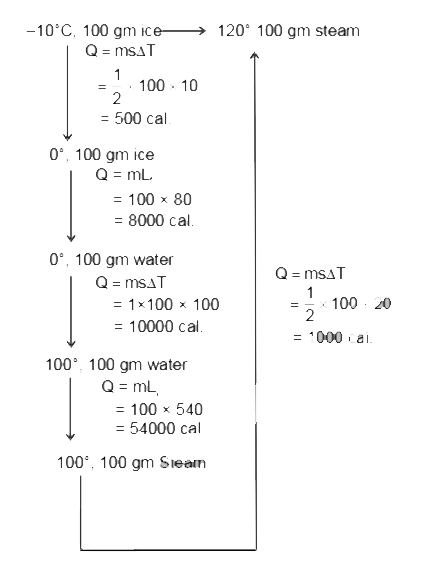
- Find amount of heat released if 100 g ice at – 10°C is converted into ...
Text Solution
|
- Heat given to a body which raises its temperature by 1^@C is
Text Solution
|
- If mass-energy equivalence is taken into account , when water is coole...
Text Solution
|
- A gaseous mixture consists of 16g of helium and 16 g of oxygen. The ra...
Text Solution
|
- If C(P and C(v) denote the specific heats nitrogen per unite mass at c...
Text Solution
|