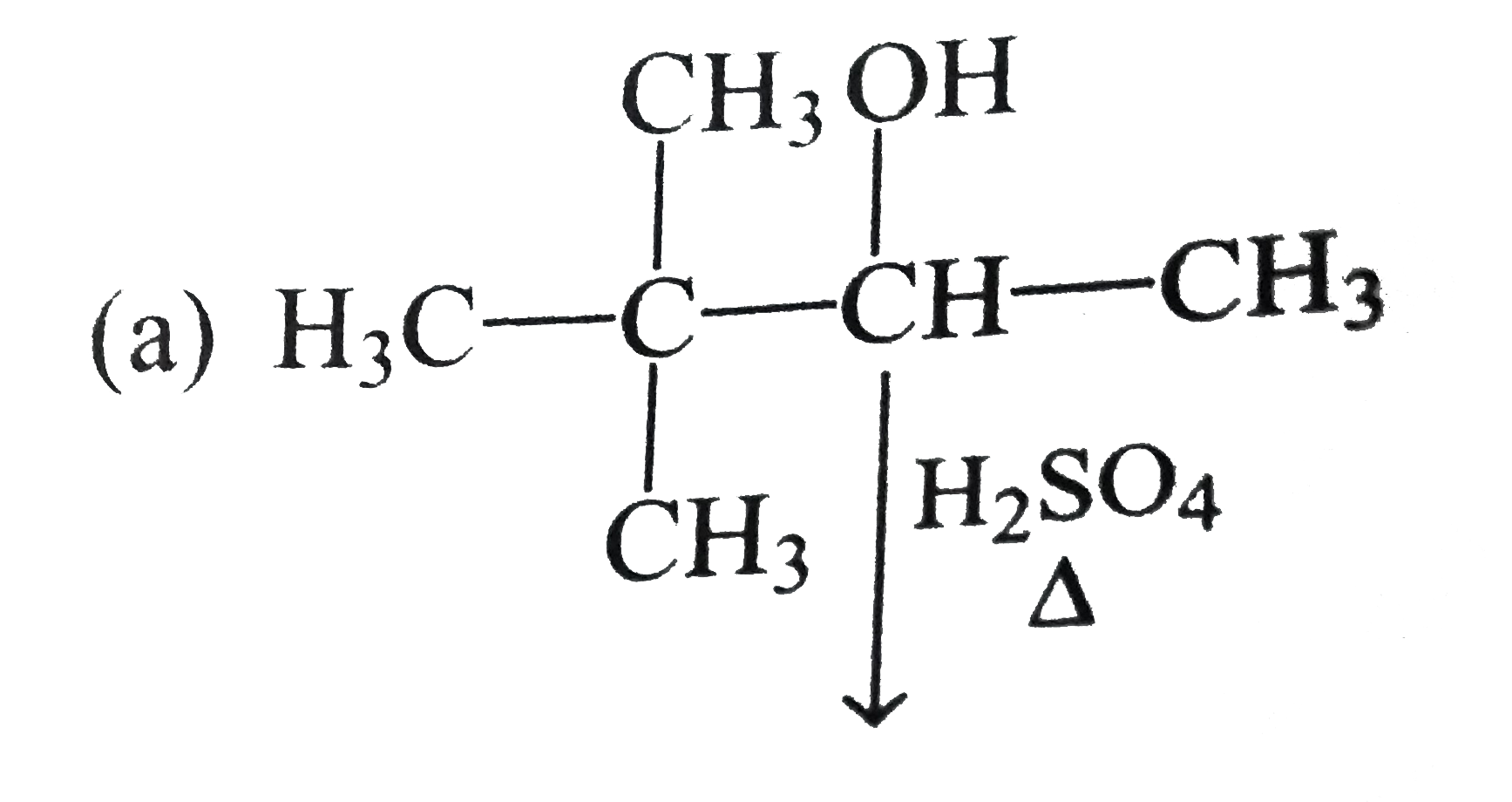
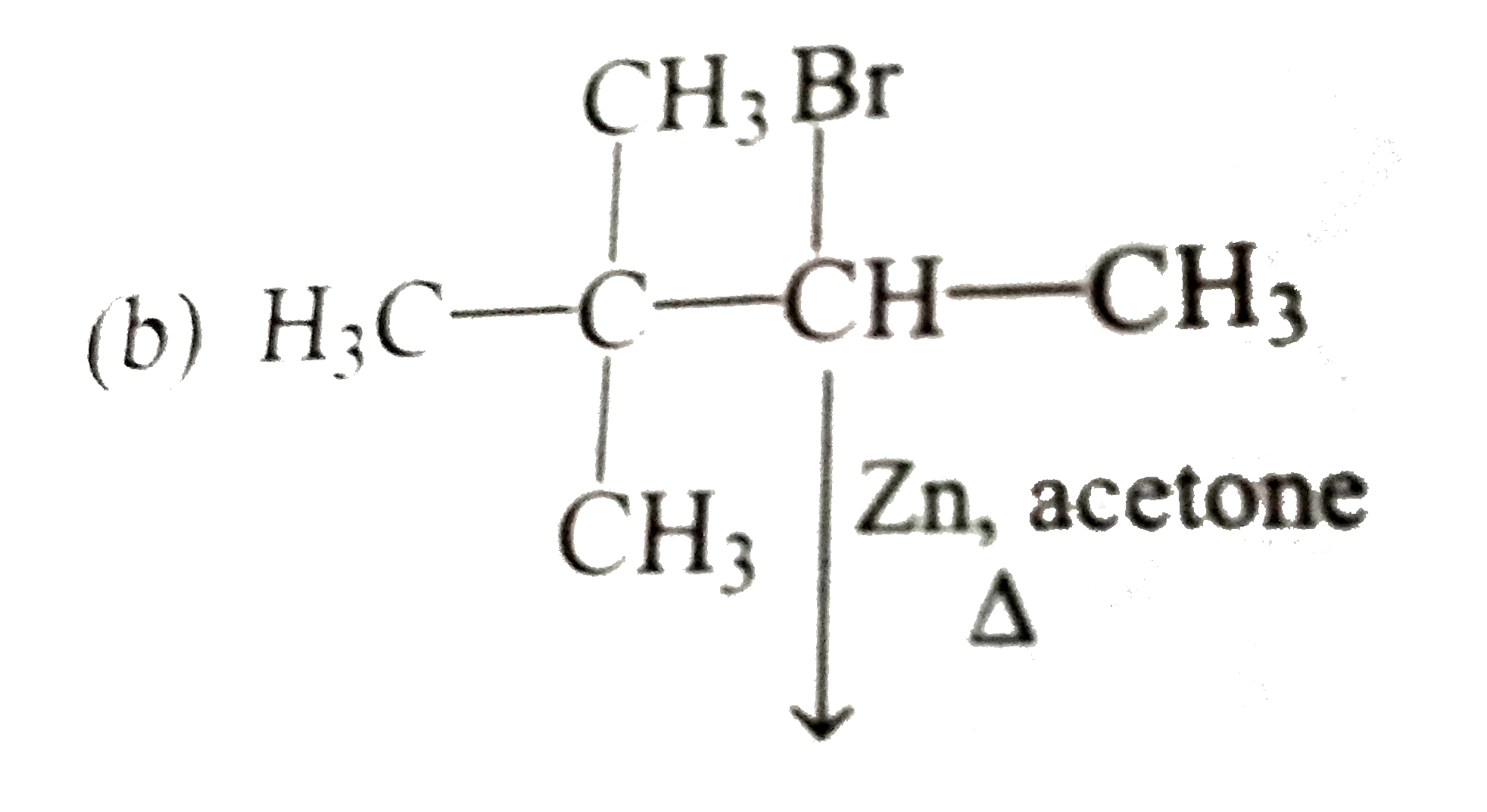
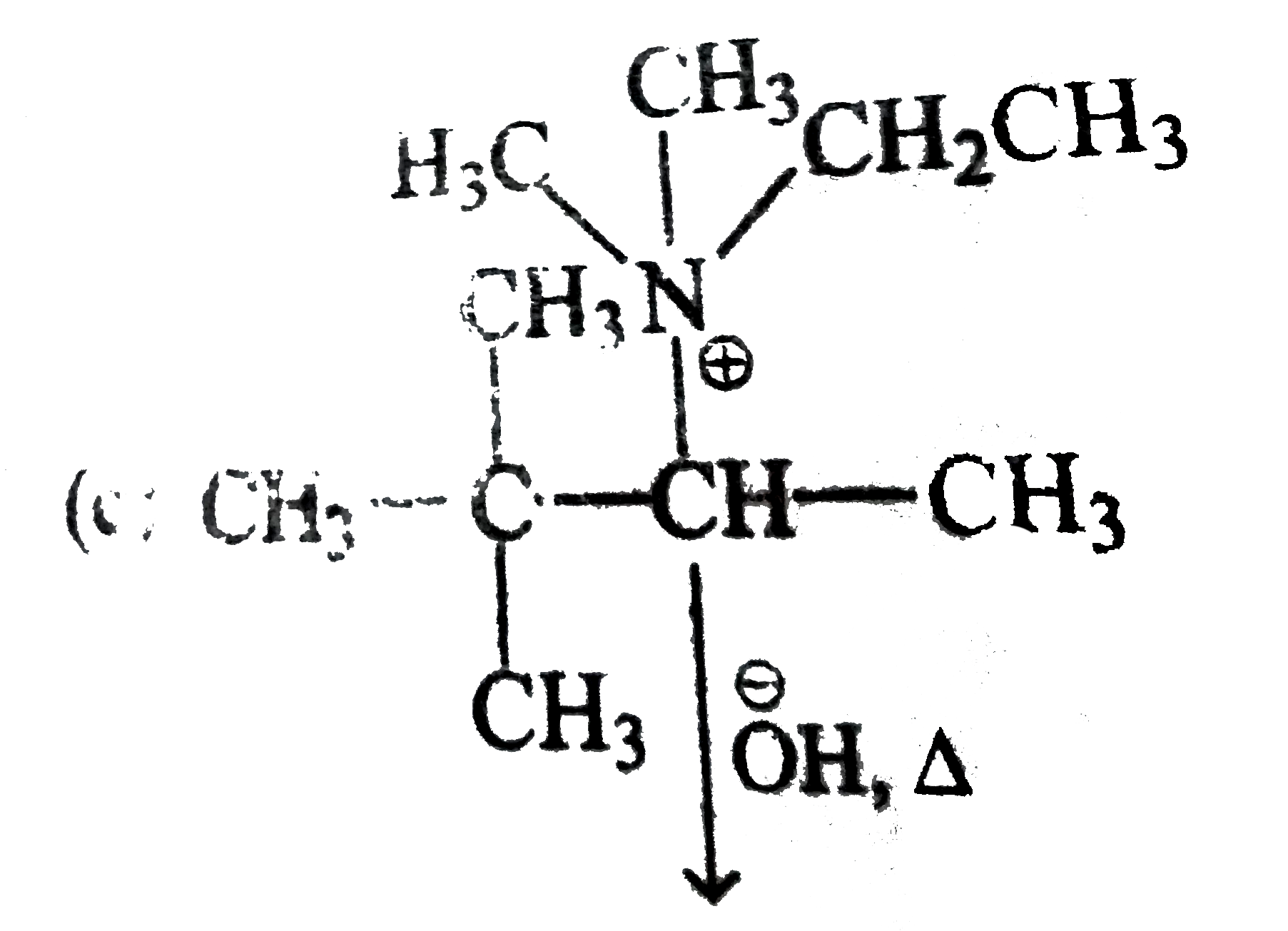
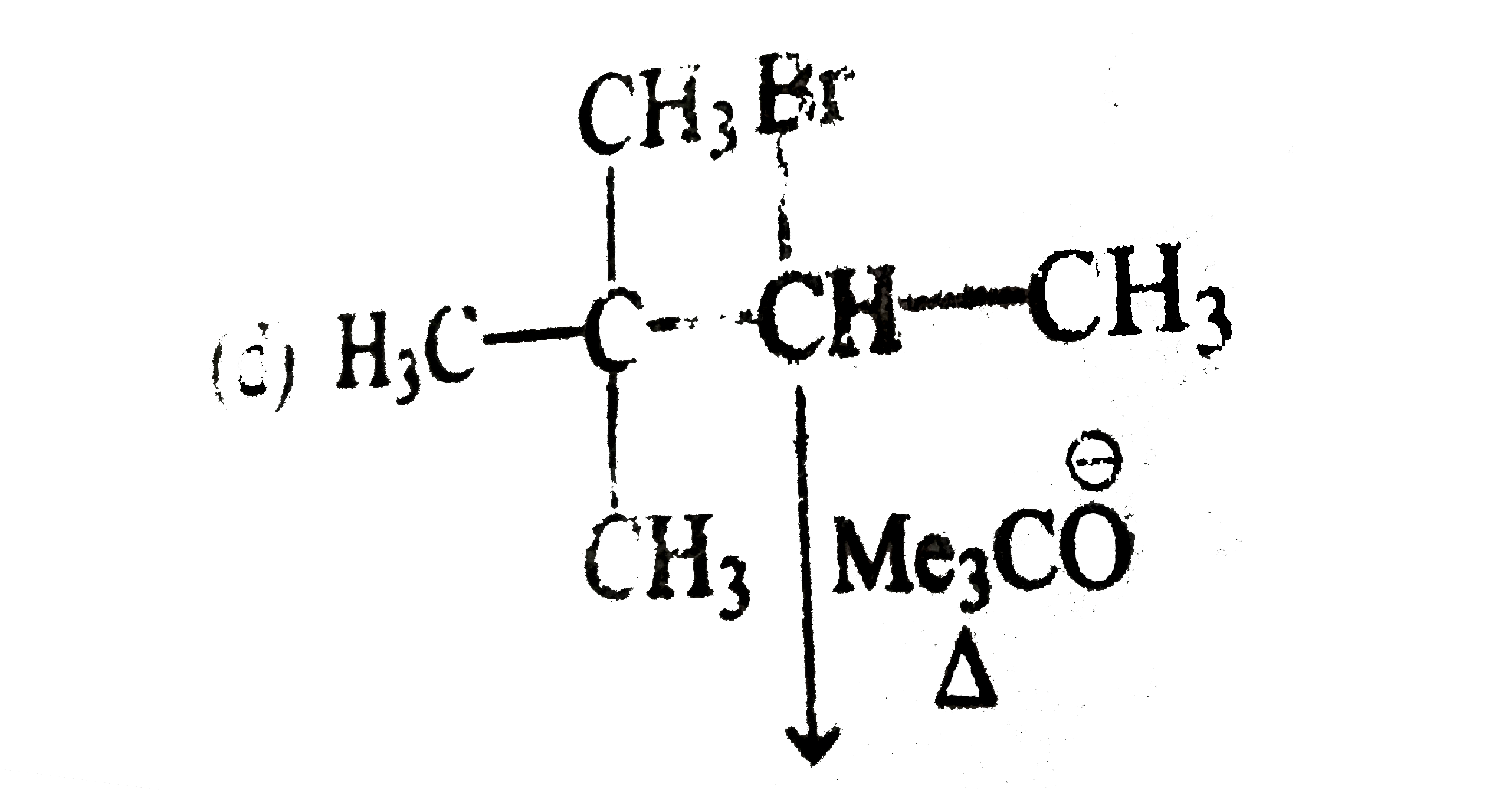A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HYDROCARBONS (ALKANE, ALKENE and ALKYNE)-Level 2 (Q.76 To Q.100)
- Propene reacts with Br(2) to give 1,2-dibromopropane.The anti addition...
Text Solution
|
- Consider the following reaction: H(3)C-overset(CH(3))overset(|)unde...
Text Solution
|
- Which of the following reactions is expected to give a fairalyt good y...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- H(2)C=CH-C=CH+HCIrarrX,X'is:
Text Solution
|
- Arrange the following reactionin decreasing order of electrophilic add...
Text Solution
|
- Complete the following reaction
Text Solution
|
- The reactivity of alkene towards hydrogen is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- A and B respectively are:
Text Solution
|
- A and B are respectively:
Text Solution
|
- What reagent is needed to acccomplish the following synthesis?
Text Solution
|
- Which compund will yield 5-keto -2 methyl hexanal upon treatment with ...
Text Solution
|
- Complete the following reaction
Text Solution
|
Text Solution
|
- H(3)C-CH=CH(2)+HCl the intermediate of reaction is:
Text Solution
|
- Choose the correct product for the following reaction :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|