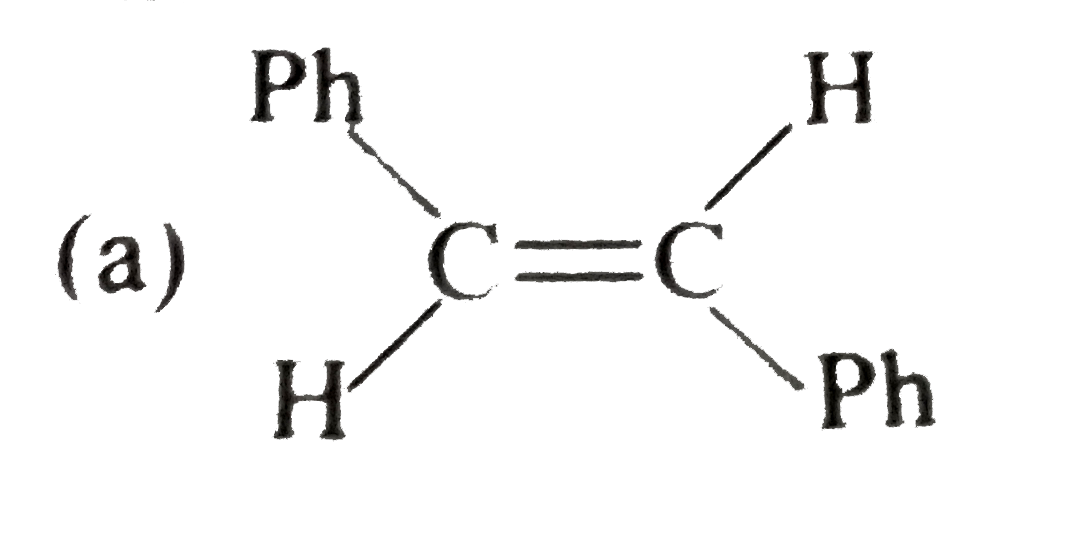
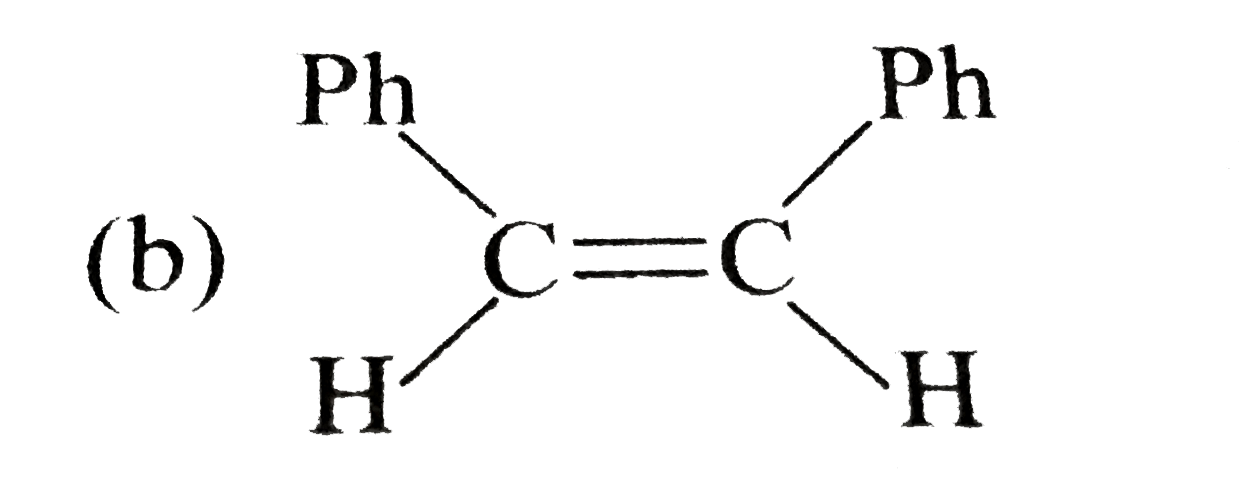
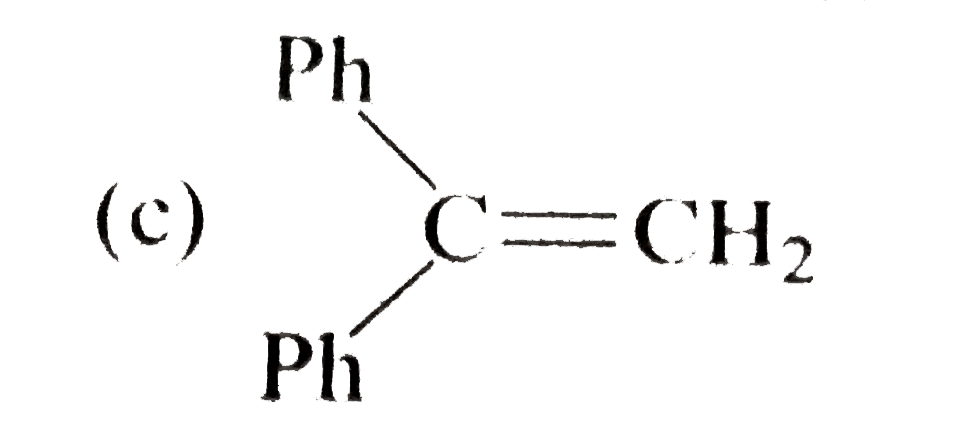
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HYDROCARBONS (ALKANE, ALKENE and ALKYNE)-Level 2 (Q.126 To Q.150)
- Stereochemistry of the product are:
Text Solution
|
- Complete the following reaction
Text Solution
|
- Ph-CH=CH=Ph underset(CCI(4))overset(CI(2))rarr X overset(2NaNH(2))rarr...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which of the following is major product of reaction shown below?
Text Solution
|
- A triene treated with ozone followed by CH(3)-S-CH(3) to give followin...
Text Solution
|
- If the following compund is treated with Pd//C in excess of H(2) gas ,...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- An organic compound C(4)H(6) on reductive ozonolysis gives HCHO,CO(2) ...
Text Solution
|
Text Solution
|
- Reagents (A) and (B) in above reaction:
Text Solution
|
- Which reaction will occur at the fastest rate?
Text Solution
|
- Complete the following reaction
Text Solution
|
- Comment on optical nature of product.
Text Solution
|
- Arrange the following compounds in decreasing order of rate of electro...
Text Solution
|
- Identify products Y and Z.
Text Solution
|
- Complete the following reaction
Text Solution
|