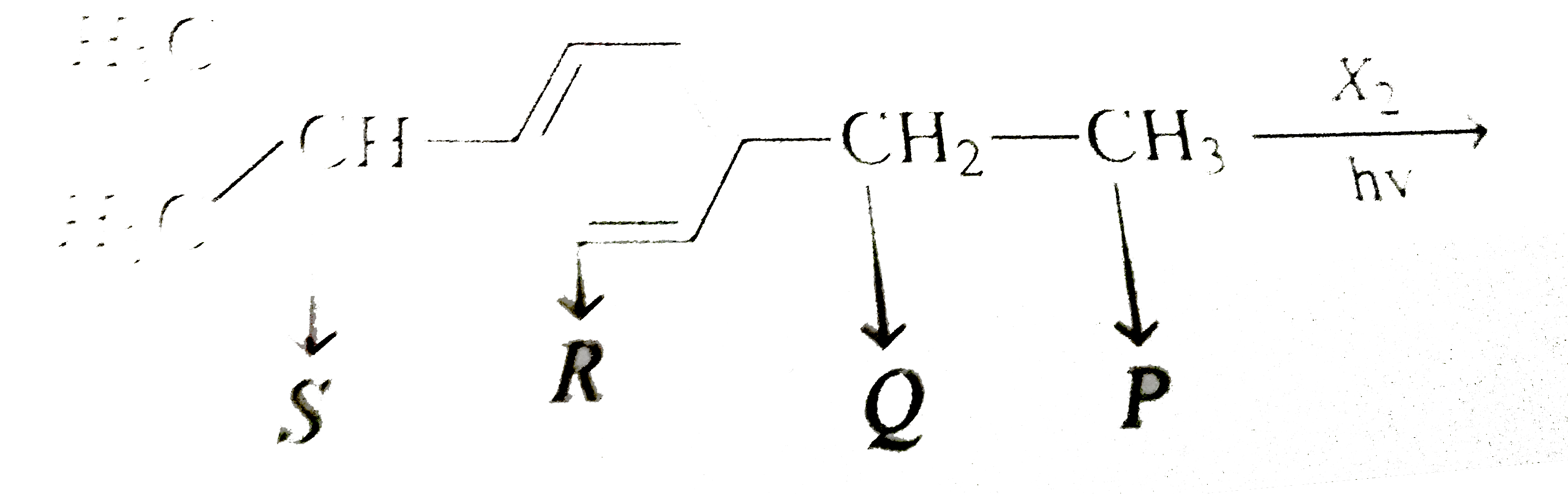
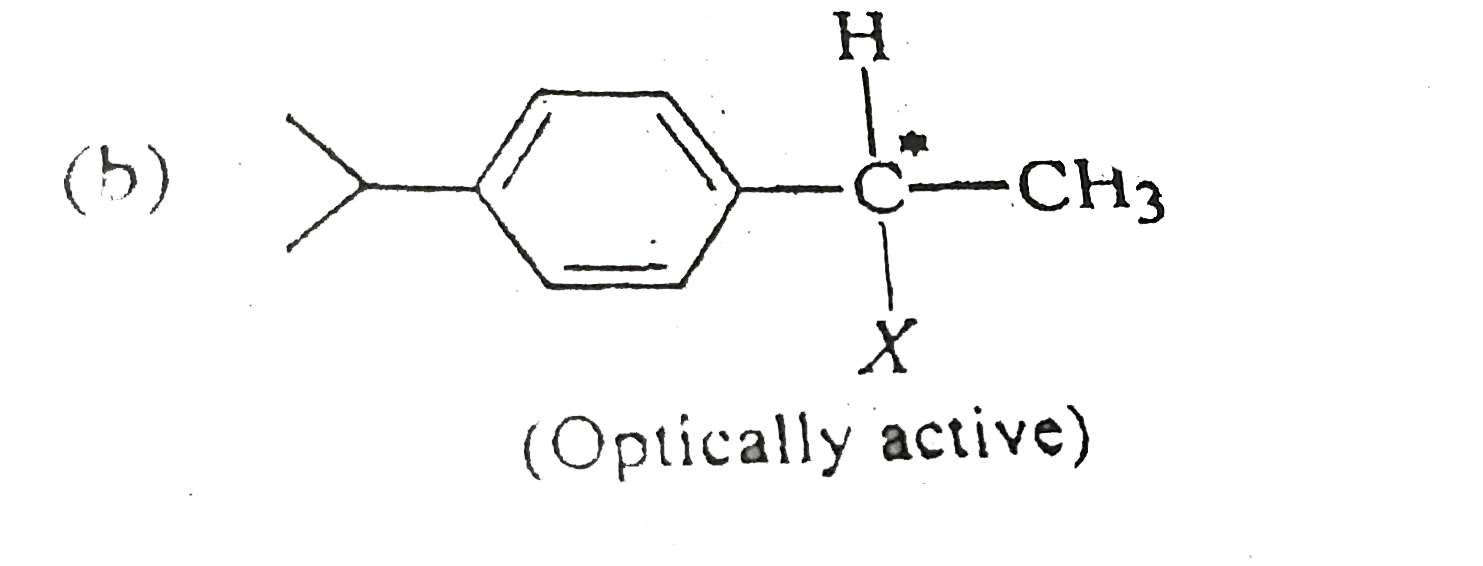
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise Match The Column|12 VideosHYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|10 VideosHYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.1 To Q.25)|25 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HYDROCARBONS (ALKANE, ALKENE and ALKYNE)-Linked Comprehension Type (Q.26 To Q.33)
- Hydrocarbon A (C(7)H(12)) was treated with BH(3) .THF, H(2)O(2), NaOH ...
Text Solution
|
- Hydrocarbon A (C(7)H(12)) was treated with BH(3) .THF, H(2)O(2), NaOH ...
Text Solution
|
- Oxymercuration demercuratioon reaction is process of additon H(2)O acc...
Text Solution
|
- Oxymercuration demercuratioon reaction is process of additon H(2)O acc...
Text Solution
|
- Oxymercuration demercuratioon reaction is process of additon H(2)O acc...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|