`S_(N^(1))` mechanism: retention of configuration:
Retention of configuration , means the starting material and product have the same configuration .
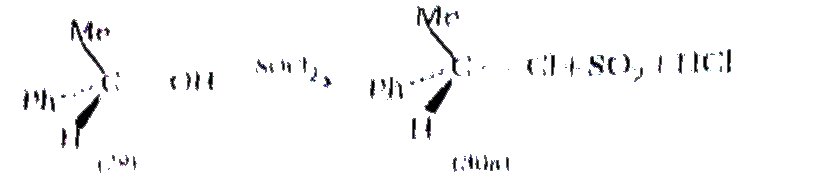
The reaction has been shown to follow a second order rate equation rate `=K_(2)` [ROH][`SOCI_(2)]`, but clearly cannot procced by the simple `S_(N^(2))` mode for this would lead to inversion of configuration in the product, which is not observed .
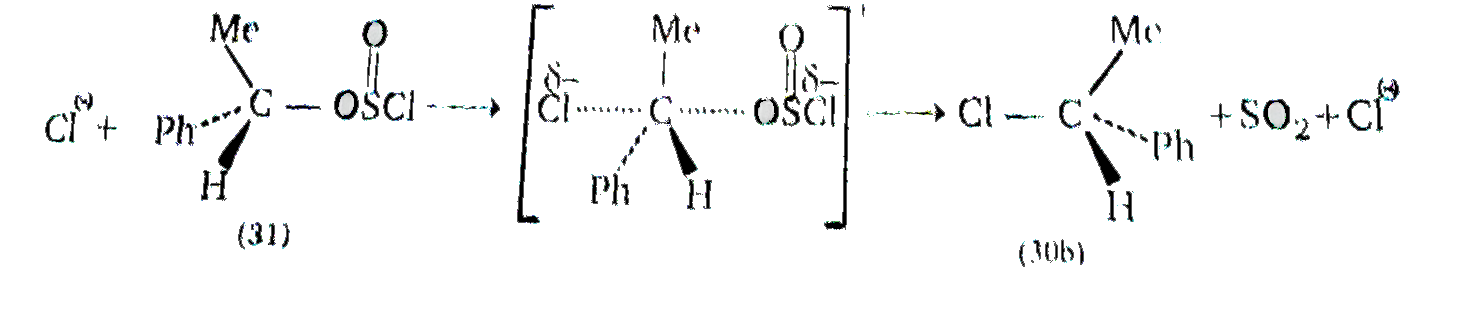
Cyrring out the reaction under milder conditions allows of the isolation of an aklyl chlorosulphite. ROSCI (31), and this can be shown to be a true intermediate. The cholorosulphite. is formed with retention of configuration , the R-o bond being broken during the reaction . The rate at which the aklyl cholrosulphite intermeidiate (31) breaks down to the product, RCI (30a) , is found to increasing polarity of the solvent , and also with increasing stability of the carbocatio `R^(o+)`: and ion pari, `R^(o+Ө)` OSOCI (32) is almost certianly involved. Provided collapse of the ion pair to products then occurs rapidly ,i.e., in the intimate ion pair (33) within a solvent cage, then attack by `CI^(Ө)` is likely to cccur on the same side of `R^(o+)` from which `^(Ө)OSCI` departed , i.e, with retention of configuration :
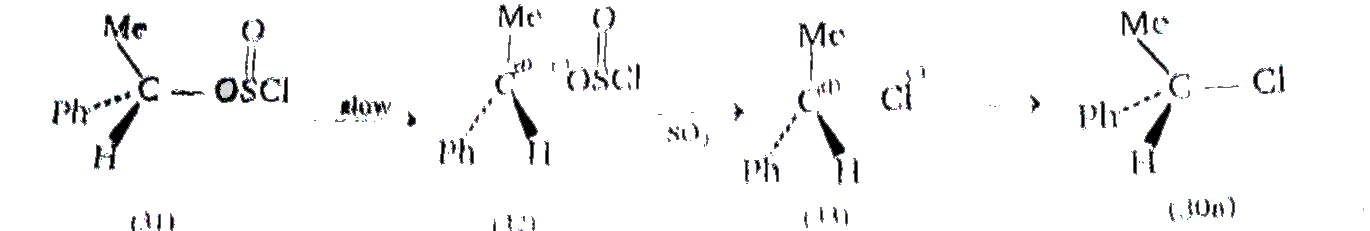
Wheather the beraking of the C-O and the S-CI bonds ocurs simultaneously, or whether the former occurs first, is stlill a matter of debate.
It is intresting that if the `SOCI_(2)` reaction on ROH (29) is carried out in hte presence of puridene, the product RCI if found now to have undrgone inversion of configuration (30b) . This ocurs because the HCI produced durning the formation of (31) from ROH and `SOCI_(2)` is conerted by pyridine into `C_(5)H_(5)NH^(o+) " " CI^(Ө) and CI^(Ө)` , being an effecting nucleophile, attacks (31) from the back in a normal `S_(N^(2))` reaction with inversion configuration :