Knowing something about the struture od a transtion state is important state is impeortant when you are tring something about the sturcture of a trasition state lies beytween the structure of the reactans and the structure of the products But what do we men by "between " ? does the strure of the tranition aterte lie exctly half way between the sturucres of the reactants and product (as in II n Below ) or does it more klike the prouctss thean the reactans (as in III)?
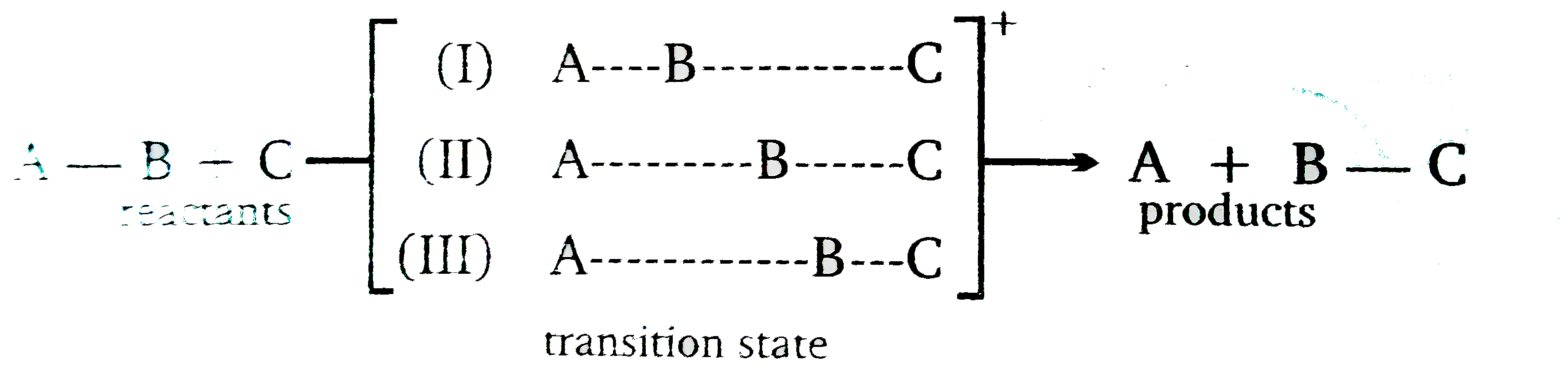
According to the transition stae will be more simlar in structure to the spcies that it is more simlar in energy t the case of an exergonuic reaction , the transition styae is more similar in energy to the reactant than to the product (curve) therfore the sturture of the trantsion state will more clostly reasemble the struutre of the reactant than that of the product In the prodect ,so the struture (curveIII) the transition statea ismore simar in enegy ot the product ,so the stucture of the rtransition state will more closely resemble energy (curveII) would we expaeract the sttusture of the transition stae to be halfway between the structuces of the reactant and the product .
Now we can underset why the tert -buty cation is formed faster then the isobuty lacation whrn 2- methypropenre reacts with HCl of the two posible products ,the terit cation (a teriarty carbocation) is more stable then the isobuty cation (a primary carbocation ) Becasuduse formaation of a carboncation is an endergonic reaction ,the sturcture of the transition state will resmble of the sturure of the carbonic reaction ,the sture ofhte transition stae will resemble the stucture of the carbocation product .this mens that the transition stae will have a singifcant amount of positve charge on a carbon .the same factor that stbilize the posittively chages product will stabilze the partially postivelty cahged transition state .therefore ,the transitin state leading to the tert -bulty cation ins more stable then the transition ste leading to the isobulty cation ,so the positive chage inthe as greast as the amount of positive charge in the transition stee is not as great asthe amount of postive change inthe carocation product , so the difference in the staiilties of hte two transition staes is not as greast afs the difference in the stabilites of the two carbocation producats.