Consider the potential energy diaram given below
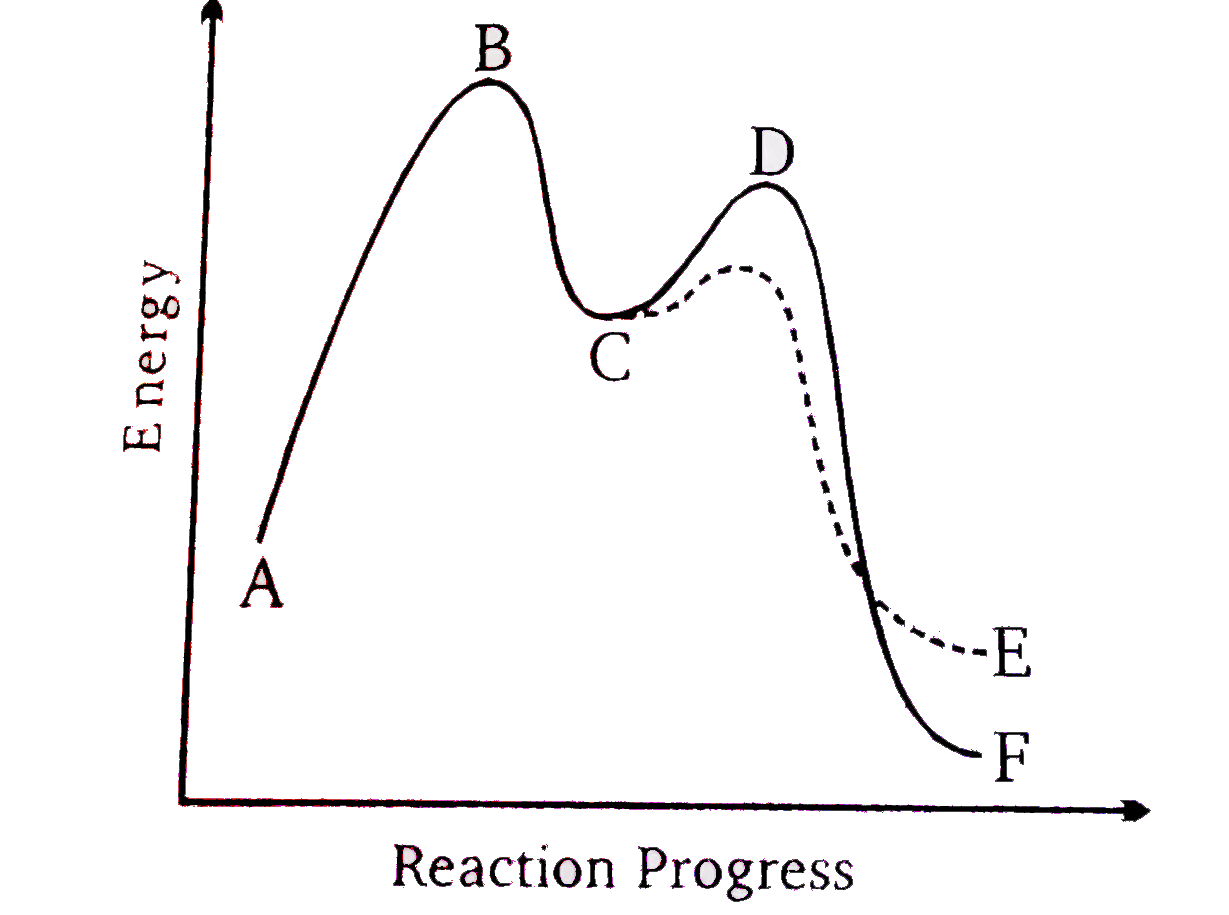
(X) Name the positions A-D
(Y) Answer the following questions .
(I) Both reaction pathways are.
(II) which step is the rate determine step?
`(III) which product is most stable ?
(iv) In accordance with Hammonds potulate , exothermixc reaction tend to have
Consider the potential energy diaram given below

(X) Name the positions A-D
(Y) Answer the following questions .
(I) Both reaction pathways are.
(II) which step is the rate determine step?
`(III) which product is most stable ?
(iv) In accordance with Hammonds potulate , exothermixc reaction tend to have

(X) Name the positions A-D
(Y) Answer the following questions .
(I) Both reaction pathways are.
(II) which step is the rate determine step?
`(III) which product is most stable ?
(iv) In accordance with Hammonds potulate , exothermixc reaction tend to have
A
Early transition states that are reasctant -like
B
Late trastion staes that are reactant -like
C
Earlty trnstion states thaat are product -like
D
Late transition states that are product-like
Text Solution
Verified by Experts
The correct Answer is:
(X) A-reactiants ,B -transition sate, C-Inter mediate ,D-transtion state (YA) (I) Exothermic (II) B(III) F(IV) A
Basic informatin.
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
Observe these compound and give answer of following questions In the given aldose which can form same osazone (I). D-Ribose (II). D-arabinose (III). D-iodose (IV). D-galactose
A chemical reaction proceeds into the following steps Step I, 2A hArrX fast Step II, X+B rarr Y slow Step III, Y+B hArr product fast The rate law for the overall reaction is
Products are favored in a chemical reaction taking place at a constant temperature and pressure. Consider the following statements : (i) The change in Gibbs energy for the reaction is negative. (ii) the total change in Gibbs energy for the reaction and the surroundings is negative. (iii) The change in entropy for the reaction is positive. (iv) The total change in entropy for the reaction and the surrounding is positive. The statments which are ALWAYS true are :
Each of the questions below consists of a question and two statements numbered I and II given below it. You have to decide whether the data provided in the statements are sufficient to answer the question. Read both the statements and give answer 1) if the data in statement I alone are sufficient to answer the question while the data in statement II alone are not sufficient to answer the question. 2) if the data in statement II alone are sufficient to answer the question while the data in statement I alone are not sufficient to answer the question. 3) if the data either in statement I alone or in statement II alone are sufficient to answer the question. 4) if the data even in both statements I and II together are not sufficient to answer the question. 5) if the data in both statements I and II together are necessary to answer the question. D is in which direction of P? I. S is to the south of P, which is to the west of D. II. P and R are in a straight line and R is to the south of D.
Study of following map and answer the questions given below: (i) Name the railway line shown in this map. (ii) In which country does this railway line lie? (iii) Name the station marked in the map as A and B.
Study the following map and answer the questions given below: (i) Name the shipping canal shown in the map. (ii) Name the Northernmost and Southern most port cities of this canal. (iii) Which two continents have made more use of this canal?
Each of the questions below consists of a question and two statements numbered I and II given below it. You have to decide whether the data provided in the statements are sufficient to answer the question. Read both the statements and give answer 1) if the data in statement I alone are sufficient to answer the question while the data in statement II alone are not sufficient to answer the question. 2) if the data in statement II alone are sufficient to answer the question while the data in statement I alone are not sufficient to answer the question. 3) if the data either in statement I alone or in statement II alone are sufficient to answer the question. 4) if the data given in both statements I and II together are not sufficient to answer the question. 5) if the data in both statements I and II together are necessary to answer the question. Z is in which direction with respect of X? I. Y is to the South of X and Z is to the East of P, which is to the North of Y. II. P is to the South of X.
Observe these compound and give answer of following questions. Q. In the given Aldose which can form same osazone (I). D-glucose (II). D-glucose (III). D-mannose (IV). D-galactose