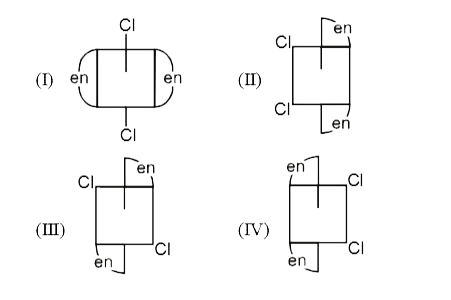
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUND
MOTION|Exercise EXERCISE -4 LEVEL I PREVIOUS YEAR JEE MAIN|20 VideosCOORDINATION COMPOUND
MOTION|Exercise EXERCISE-4 LEVEL-II PREVIOUS YEAR JEE ADVANCED|37 VideosCOORDINATION COMPOUND
MOTION|Exercise EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED Match the Column|10 VideosCLASSROOM PROBLEMS 1
MOTION|Exercise THERMODYNAMICS|17 VideosD-BLOCK ELEMENTS
MOTION|Exercise Exercise IV Level-II|9 Videos
Similar Questions
Explore conceptually related problems
MOTION-COORDINATION COMPOUND-EXERCISE -3 SUBJECTIVE JEE ADVANCED
- Which of the following complexes shows ionization isomerism ?
Text Solution
|
- The number of possible isomers by the compounds like [Cd(gly)2] and [P...
Text Solution
|
- Identify the geometrical isomers of the following
Text Solution
|
- When [Ni(NH(3))(4)]^(2+) is treated with conc HCI, two compounds havin...
Text Solution
|
- Which of the following isomers of [M(NH3)2Cl2] would react with potass...
Text Solution
|
- Which of the following octahedral complex does not show geometrical is...
Text Solution
|
- Octahedral complex of Ni(II) will be always:
Text Solution
|
- For the correct assignment of electronic configuration of a complex, t...
Text Solution
|
- Mn^(2+) forms a complex with Br– ion. The magnetic moment of the compl...
Text Solution
|
- [Co(H2O)6]^(3+) and [PdBr4]^(2–) complex ions are respectively :
Text Solution
|
- The species having tetrahedral shape is
Text Solution
|
- Point out the correct statements amongst the following :
Text Solution
|
- Among the following ions which one has the highest paramagnetism?
Text Solution
|
- Among the following, the compound that is both paramagnetic and colour...
Text Solution
|
- A complex of certain metal has the magnetic moment of 4.91 BM whereas ...
Text Solution
|
- Which of the following statements are true/false (i) [Co(H2O)4]^(2+)...
Text Solution
|
- All the following complexes show decrease in their weights when placed...
Text Solution
|
- What is oxidation state, magnetic moment and type of hybridisation of ...
Text Solution
|
- Which of the following species have maximum number of unpaired elect...
Text Solution
|
- Which of the following complexes is diamagnetic ?
Text Solution
|