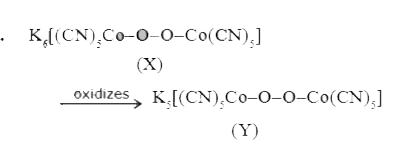A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUND
MOTION|Exercise EXERCISE -4 LEVEL I PREVIOUS YEAR JEE MAIN|20 VideosCOORDINATION COMPOUND
MOTION|Exercise EXERCISE-4 LEVEL-II PREVIOUS YEAR JEE ADVANCED|37 VideosCOORDINATION COMPOUND
MOTION|Exercise EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED Match the Column|10 VideosCLASSROOM PROBLEMS 1
MOTION|Exercise THERMODYNAMICS|17 VideosD-BLOCK ELEMENTS
MOTION|Exercise Exercise IV Level-II|9 Videos
Similar Questions
Explore conceptually related problems
MOTION-COORDINATION COMPOUND-EXERCISE -3 SUBJECTIVE JEE ADVANCED
- Among the following ions which one has the highest paramagnetism?
Text Solution
|
- Among the following, the compound that is both paramagnetic and colour...
Text Solution
|
- A complex of certain metal has the magnetic moment of 4.91 BM whereas ...
Text Solution
|
- Which of the following statements are true/false (i) [Co(H2O)4]^(2+)...
Text Solution
|
- All the following complexes show decrease in their weights when placed...
Text Solution
|
- What is oxidation state, magnetic moment and type of hybridisation of ...
Text Solution
|
- Which of the following species have maximum number of unpaired elect...
Text Solution
|
- Which of the following complexes is diamagnetic ?
Text Solution
|
- The complex having highest Delta value
Text Solution
|
- For the t(2g)^6eg^2 system, the value of magnetic moment (mu) is:
Text Solution
|
- Which of the following complex is with lowest number of unpaired elect...
Text Solution
|
- The complex K(4)[Zn(CN)(4)(O(2))(2)] is oxidised into K(2)[Zn(CN)(4)(O...
Text Solution
|
- In both the complexes Co cation have t(2g)^6 eg^0 configuration. The...
Text Solution
|
- Which one of the following species does not represent cationic species...
Text Solution
|
- Following Sidgwick's rule of EAN, Co(CO)(x) will be.
Text Solution
|
- In the Iso-electronic series of metal carbonyls, the C-O bond strength...
Text Solution
|
- The magnetic moment of green complex is 1.7 BM & for brown complexes m...
Text Solution
|
- If NO reacts with [Cr(CO)6] how many CO groups can be replaced by NO :
Text Solution
|
- The value of the magnetic moment of a particular ion is 2.83 Bohr magn...
Text Solution
|
- The complex ion . [M(en)Br(2)I(2)]^(-1), has two optical isomers. Thei...
Text Solution
|