Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE -EXERCISE-4 (LEVEL-II)
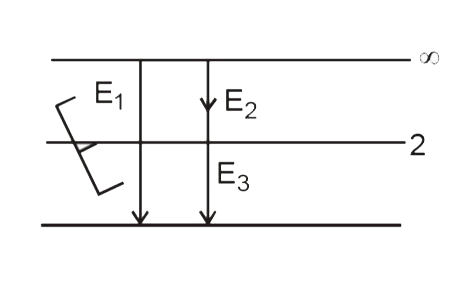
- Let nu(1) be the frequency of the series limit of the lyman series n...
Text Solution
|
- हाइड्रोजन परमाणु में इलेक्ट्रॉन के n = 1 कक्षा से n = 2 कक्षा में जाने...
Text Solution
|
- The minimum orbital angular momentum of the electron in a hydrogen ato...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- Energy of the state S(1) in units of the hydrogen atom ground state en...
Text Solution
|
- The orbital angular momentum quantum number of the state S(2) is
Text Solution
|
- The maximum number of electrons that can have principal quantum number...
Text Solution
|
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- The work function of Silver and sodium are 4.6 and 2.3 eV, respective...
Text Solution
|
- The radius of the orbit of an electron in a Hydrogen-like atom is 4.5 ...
Text Solution
|
- Consider the follwing complexes ion P,Q and R P =[FeF(6)]^(3-), Q=[V...
Text Solution
|
- In an atom, the total number of electrons having quantum numbers n =...
Text Solution
|
- Energy of an electron is givem by E = - 2.178 xx 10^-18 J ((Z^2)/(n^2)...
Text Solution
|
- Not considering the electronic spin, the degeneracy of the second exci...
Text Solution
|
- P is the probability of finding the Is electron of hydrogen atom in a ...
Text Solution
|
- Wave function psi(n,lm(1)) is a mathematical function, which vlaue dep...
Text Solution
|
- Wave function psi(n,lm(1)) is a mathematical function, which vlaue dep...
Text Solution
|
- Wave function psi(n,lm(1)) is a mathematical function, which vlaue dep...
Text Solution
|