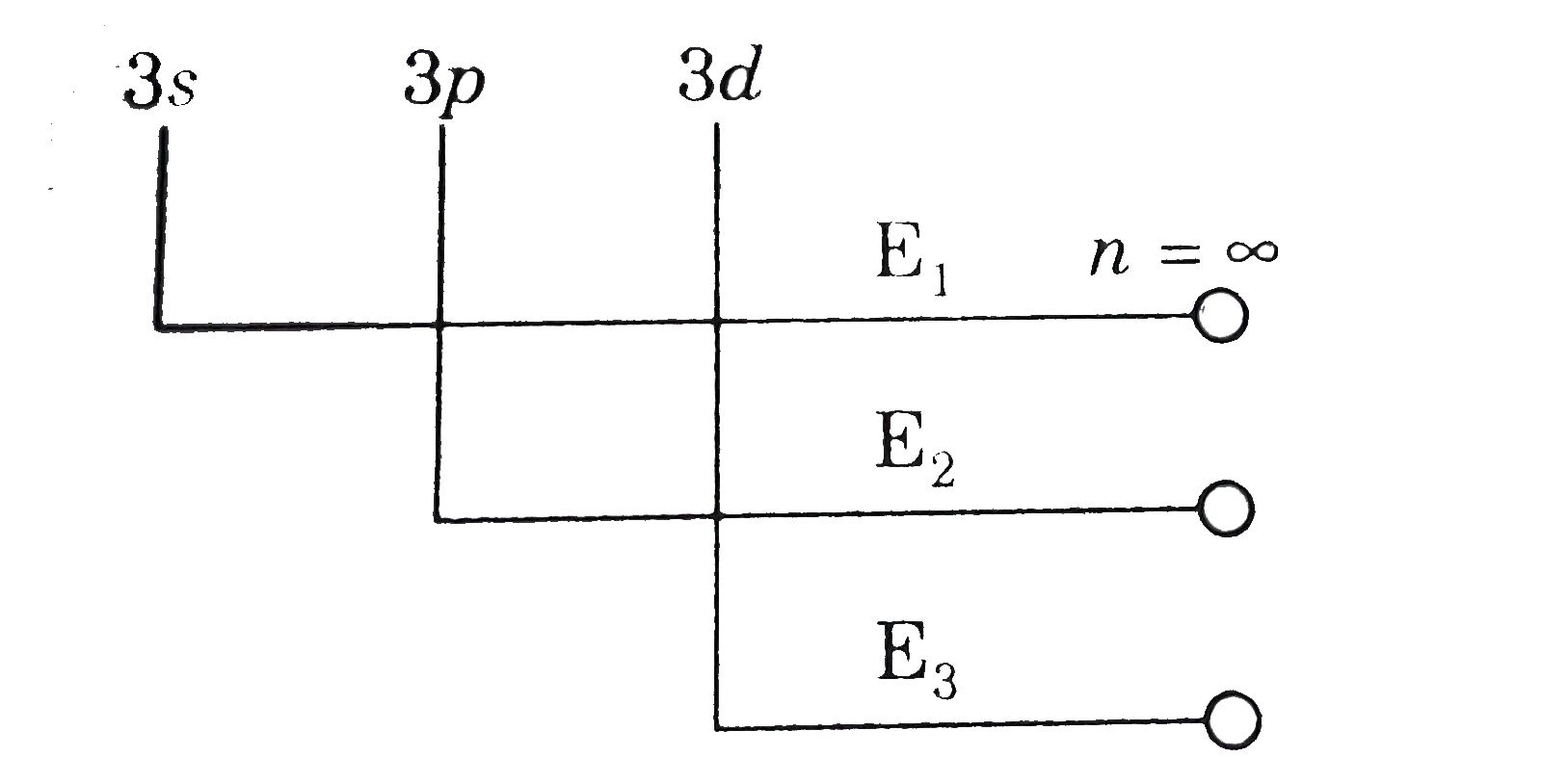
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
MOTION|Exercise EXERCISE-2 (LEVEL-I)|53 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-2 (LEVEL-II)|31 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-II)|18 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-II|16 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 4 (LEVEL II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE -EXERCISE-1
- A gas absorbs a photon of 300 nm and then reemitts two photons. One ph...
Text Solution
|
- The specific charge of a proton is 9.6 xx 10^7 C kg^-1, then for and a...
Text Solution
|
- For H-atoms , the energy required for the removal of electron from var...
Text Solution
|
- The wave number of the first line of Balmer series of hydrogen is 15...
Text Solution
|
- The wavelength of the third line of the Balmer series for a hydrogen a...
Text Solution
|
- Wave number of spectral line for a given transition is x cm^(-1) for H...
Text Solution
|
- A photon was absorbed by a hydrogen atom in its ground state and the e...
Text Solution
|
- Wavelength of the first line of Paschen series is - (R = 109700 cm^-1)
Text Solution
|
- Which of the following is responsible to rule out the existence of def...
Text Solution
|
- Which of the following expressions represents the spectrum of Balmer s...
Text Solution
|
- The number of unpaired electrons in Zn^(2+) is
Text Solution
|
- The number of orbitals in 3rd orbit are
Text Solution
|
- If the number of electrons in p-orbital are two, then which one of the...
Text Solution
|
- The number of electrons in Na, having n+l =3
Text Solution
|
- Which orbital has 1 nodal plane -
Text Solution
|
- which of the following is correct set of quantum numbers for the last...
Text Solution
|
- The quantum number not obtained from the schrodinger's wave equation i...
Text Solution
|
- The total number of electrons in a principal energy shell is designate...
Text Solution
|
- Which of the following statements cocerning the four quantum numbers i...
Text Solution
|
- How many electrons can fit into the orbitals that comprise the 3^(rd) ...
Text Solution
|