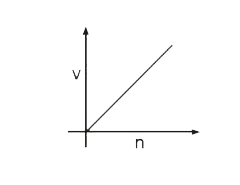
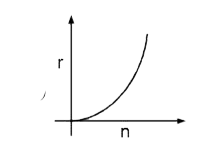
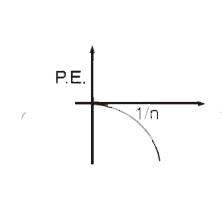
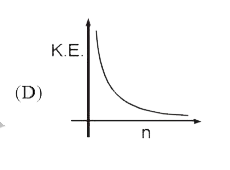
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
MOTION|Exercise EXERCISE-3|30 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-I)|16 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-2 (LEVEL-I)|53 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-II|16 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 4 (LEVEL II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE -EXERCISE-2 (LEVEL-II)
- In photoelectric effect, stopping potential depends on
Text Solution
|
- In the experiment on photoelectric effect using light having frequency...
Text Solution
|
- Select the correct curve(s) : If v = velocity of electron in Bohr’s ...
Text Solution
|
- Select the correct statement(s) :
Text Solution
|
- Which is/are correct statement.
Text Solution
|
- Statement -I : Energy emitted when an electron jump from 5rarr2 (ener...
Text Solution
|
- Satement-1: Emitted radiation will fall in visible range when an elect...
Text Solution
|
- In an excited state of hydrogen like atom an electron has total energy...
Text Solution
|
- Choose the correct statement among the following:
Text Solution
|
- Correct statement (s) regarging 3p(y) orbital is//are :
Text Solution
|
- Choose the incorrect statement(s) :
Text Solution
|
- Assertion : The fraction of total number of electrons in p-sub levels ...
Text Solution
|
- Assertion : The energy of an electron is mainly determined by principa...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- A single electron orbits around a stationary nucleus of charge +Ze, wh...
Text Solution
|
- A single electron orbits around a stationary nucleus of charge +Ze, wh...
Text Solution
|
- A single electron orbits a stationary nucleus of charge + Ze, where Z ...
Text Solution
|