A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-MOLE CONCEPT-EXERCISE - 2 (LEVEL - I)
- Which has maximum number of atoms of oxygen
Text Solution
|
- The number of atoms present in 0.5 moles of nitrogen is same as the at...
Text Solution
|
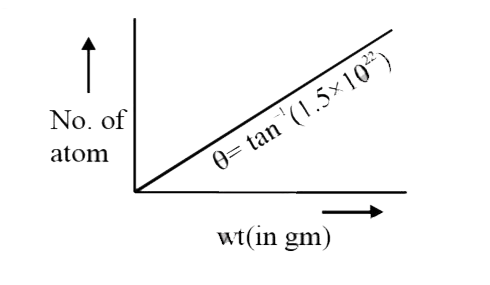
- A graph is plotted for an element, by putting its weight on X-axis a...
Text Solution
|
- Two elemets X( atomic weight =75) and Y( atomic weight =16) combine to...
Text Solution
|
- The pair of species having same percentage of carbon is:
Text Solution
|
- An iodized salt contains 0.5% of Nal. A person consumes 3 gm of salt e...
Text Solution
|
- In a textile mill, a double-effect evaporator system concentrates weak...
Text Solution
|
- The empirical formula of a compound of molecular mass 120 is CH(2)O. ...
Text Solution
|
- Calcualte the molecualr formula of compound which contains 20% Ca and ...
Text Solution
|
- The vapour density of a mixture of gas A (Molecular mass = 40) and gas...
Text Solution
|
- Average atomic mass of magnesium is composed of 79 mole % of .^(2...
Text Solution
|
- The moles of O(2) required for reacting with 6.8 gm of ammonia. (4NH...
Text Solution
|
- If one mole of ethanol (C(2)H(5)OH) completely burns to form carbon di...
Text Solution
|
- According to the following reaction the minimum quantity in gm of H(2)...
Text Solution
|
- The % loss in weight heating a pure sample of potassium chlorate (M. w...
Text Solution
|
- When 2.76 g of silver carbonate is strongly heated, it yields a residu...
Text Solution
|
- PH(3)(g) decomposes on heating to produce phosphorous and hydrogen. Th...
Text Solution
|
- The mass of 70% H(2)SO(4) required for neutralization of one mole of N...
Text Solution
|
- Calculate the amount of Ni needed in the Mond's process given below ...
Text Solution
|
- For the reaction 2x+3y+4z rarr 5w Initially if 1 mole of x, 3 mole...
Text Solution
|