Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-BIOMOLECULES & POLYMERS-EXERCISE - 4 (LEVEL II)
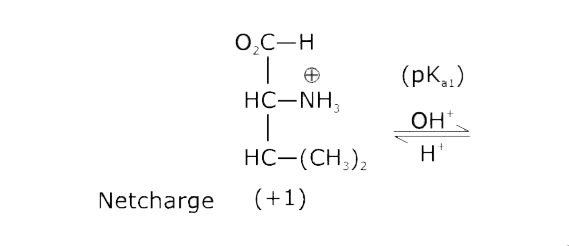
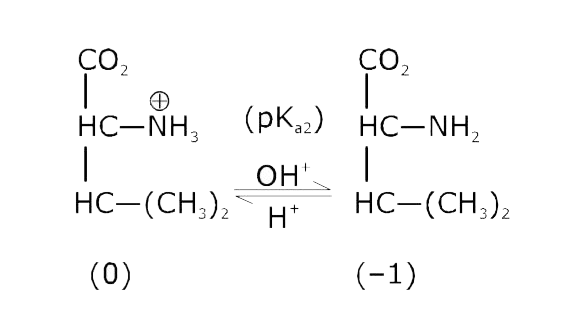
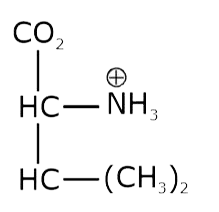
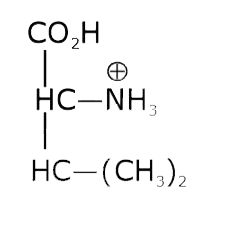
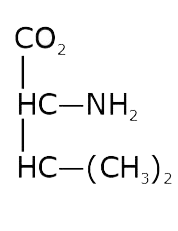
- The pKa of the carboxyl group in an amino acid valine, (CH3)2 CHCH(NH2...
Text Solution
|
- Which of the following pairs give positive Tollen's test ?
Text Solution
|
- The two forms of D-glucopyranose obtained from solution of D-glucose a...
Text Solution
|
- Match the chemical substances in Column I with type of polymers/type o...
Text Solution
|
- Cellulose upon acetylation with excess acetic anhydride/H(2)SO(4) (cat...
Text Solution
|
- Among cellulose, poly (vinyl chloride), nylon and natural rubber, the ...
Text Solution
|
- The correct statement(s) about the following sugars (X) and (Y) is//ar...
Text Solution
|
- The correct statement about the following disaccharide is
Text Solution
|
- The total number of basic groups in the following form of lysine is
Text Solution
|
- The following carbohydrate is
Text Solution
|
- The substituents R(1) and R(2) for nine peptides are listed in the tab...
Text Solution
|
- The total number of distinct naturally occuring amino acids obtained b...
Text Solution
|
- The total number of lone-pairs of electrons in melamine is.
Text Solution
|
- A tetrapeptide has -COOH group on alanine. This produces glycine (Gly)...
Text Solution
|
- The structure of D-(+)- glucose is The structure of L-(-) glucose...
Text Solution
|
- For 'invert sugar' the correct statement is (Given : specific rotati...
Text Solution
|
- On complete hydrogenation, natural rubber produces
Text Solution
|
- The Fischer presentation of D-glucose is given below. the correct...
Text Solution
|