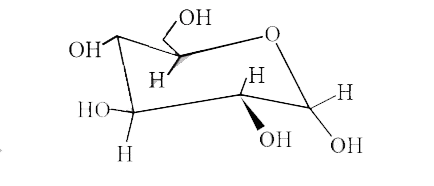
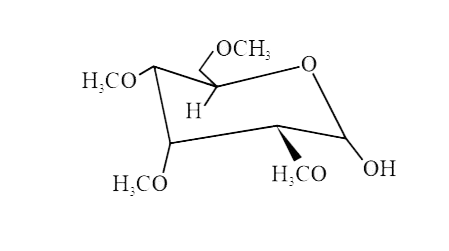
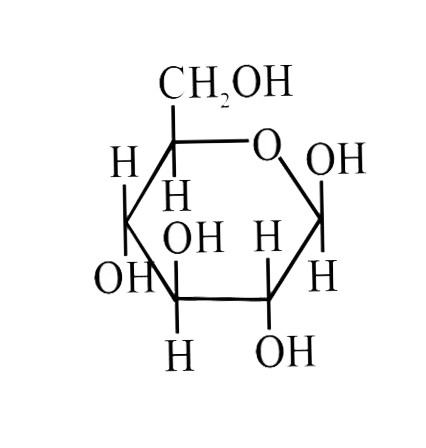
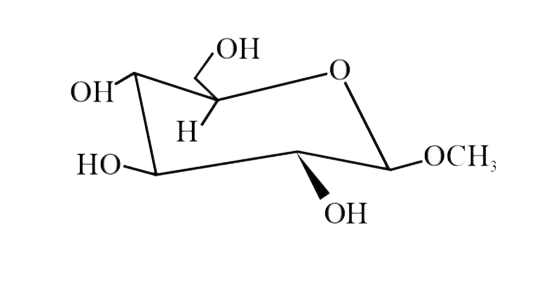
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 2 ( LEVEL - II SECTION B)|19 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 2 ( LEVEL - II SECTION C)|19 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 2 ( LEVEL - I SECTION E)|20 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-II)|18 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 4 LEVEL -II|24 Videos
Similar Questions
Explore conceptually related problems
MOTION-BIOMOLECULES & POLYMERS-EXERCISE - 2 ( LEVEL - II SECTION A)
- Which one of the following is non-reducing sugar ?
Text Solution
|
- Which of the following is/are reducing sugar
Text Solution
|
- In solution glucose exist in how many isomeric forms?
Text Solution
|
- Reducing property of monosaccharide is due to the presence of
Text Solution
|
- Which one of the following carbohydrates will show mutarotation?
Text Solution
|
- Glucose and mannose are :
Text Solution
|
- Which of the following pairs are epimers
Text Solution
|
- Glucose +x phenyl hydrazine to osazone 'x' will be :
Text Solution
|
- Which of the following reagent can distinguish between glucose and fru...
Text Solution
|
- Cellulose is a polymer of
Text Solution
|
- Which of the following is an example of ketohexose?
Text Solution
|
- Complete hydrolysis of cellulose gives:
Text Solution
|
- Total number of chiral carbons in beta-D(+) glucose is
Text Solution
|
- Which of the following compounds give positive test with Tollen's reag...
Text Solution
|
- Among cellulose, poly (vinyl chloride), nylon and natural rubber, the ...
Text Solution
|
- What is the relation between D-glucose and L-glucose? Among D-glucose ...
Text Solution
|
- Identify the pair of Epimers
Text Solution
|
- The number of Stereogenic centres in alpha-D-Glucose are
Text Solution
|
- alpha-D glucose and beta-D -glucose are
Text Solution
|
- A non-reducing sugar is
Text Solution
|