A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
MOTION|Exercise Exercise - 3 | Subjective | JEE Advanced|53 VideosP-BLOCK ELEMENTS
MOTION|Exercise Exercise - 4 | Level-I Previous Year | JEE Main|37 VideosP-BLOCK ELEMENTS
MOTION|Exercise Exercise - 1 Objective Problems | JEE Main|45 VideosOXIDATION & REDUCTION
MOTION|Exercise EXERCISE -4(LEVEL-II)|10 VideosPERIODIC TABLE
MOTION|Exercise Exercise - 4 | Level-II|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-P-BLOCK ELEMENTS-Exercise - 2 Objective Problems | JEE Adv.
- Borax is actually made of two tetrahedra and two triangular units join...
Text Solution
|
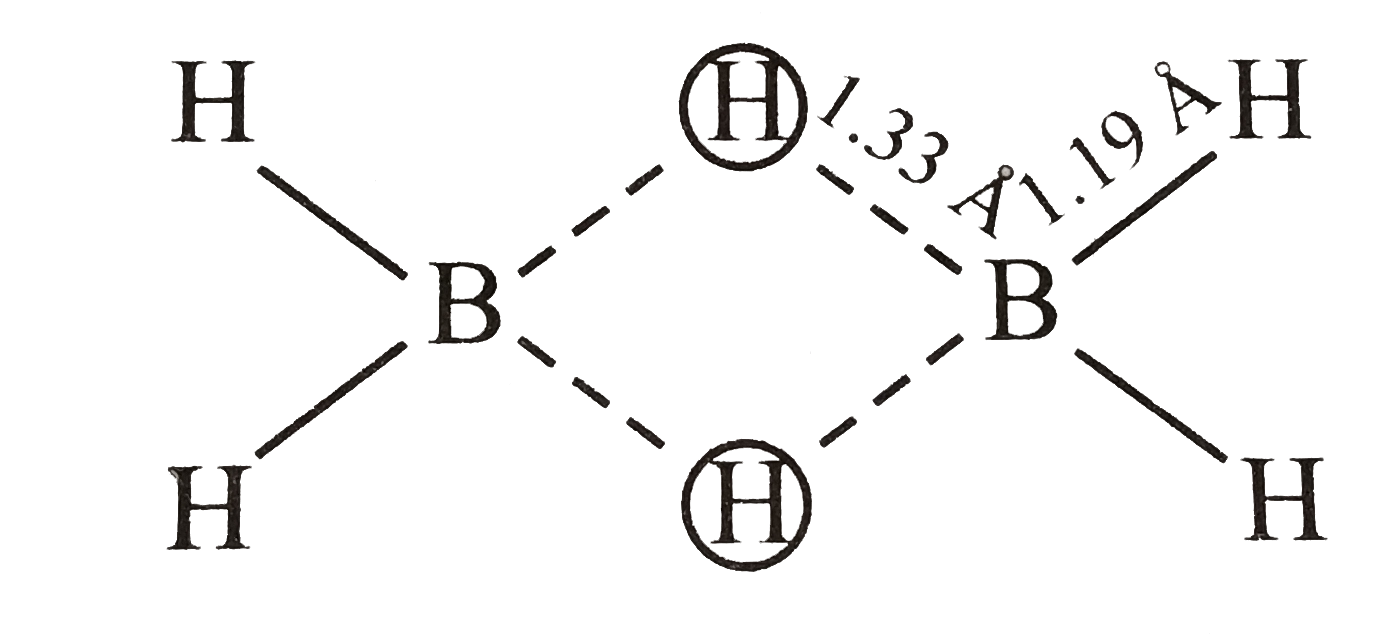
- The molecular shapes of diborane is shown below : . Consider the f...
Text Solution
|
- How can the following reaction be made to proceed in forward direction...
Text Solution
|
- In which of the following, a salt of the type KMO(2) is obtained ?
Text Solution
|
- Read name silica covers an entire group of minereal, which have the ge...
Text Solution
|
- Read name silica covers an entire group of minereal, which have the ge...
Text Solution
|
- Read name silica covers an entire group of minereal, which have the ge...
Text Solution
|
- Silicones are synthetic polymers containing repeated R2 SiO units . Si...
Text Solution
|
- Silicones are sunthetic polyners conitainging repeated R2 SiO units . ...
Text Solution
|
- The general formula of cyclic or ring silicates is
Text Solution
|
- Silica reacts with magnesium to form magnesium compound (X).(X) reacts...
Text Solution
|
- Silica is reacted with sodium carbonate . What is the gas liberateed ?
Text Solution
|
- A bottle of fire exitingushers contain H(2)SO(4) and:
Text Solution
|
- The following flow diagram represented the industrial preparatioin of ...
Text Solution
|
- The following flow diagram represented the industrial preparatioin of ...
Text Solution
|
- There are some deposits of nitrated and phosphates in the earth's crus...
Text Solution
|
- There are some deposits of nitrated and phosphates in the earth's crus...
Text Solution
|
- White phosphorus on reaction with NaOH gives PH(3) as one of the produ...
Text Solution
|
- Which one of the oxides of nitrogen dimerises into colourless solid/li...
Text Solution
|
- Ammonia reaxcts with Nessler's reagent to give:
Text Solution
|
 .
.