A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-SURFACE CHEMISTRY-Exercise - 3 (Level-II)
- One gram of charcoal adsorbs 100 mL of 0.5 M CH(3)COOH to form a mono-...
Text Solution
|
- Around 20% surface sites have adsorbed N(2). On heating N(2) gas evolv...
Text Solution
|
- Assertion (A): Micelles are formed by surfactant molecules above the c...
Text Solution
|
- Among the following the surfactant that will from micelles in squeous ...
Text Solution
|
- Among the electrolytes Na(2),SO(4),CaCl(2),Al(2)(SO(4))(3) and NH(4)Cl...
Text Solution
|
- Silver (atomic weight 108 g mol^(-1)) has a density of 10.5 g cm^(-3)....
Text Solution
|
- The correct statement(s) pertaining to the adsorption of a gas on a so...
Text Solution
|
- The given graph/data I,II,III and IV represent general trends observe...
Text Solution
|
- Choose the correct reason(s) for the stability of the lyophobic colloi...
Text Solution
|
- Methylene blue, from its aqueous solution, is adsorbed on activated ch...
Text Solution
|
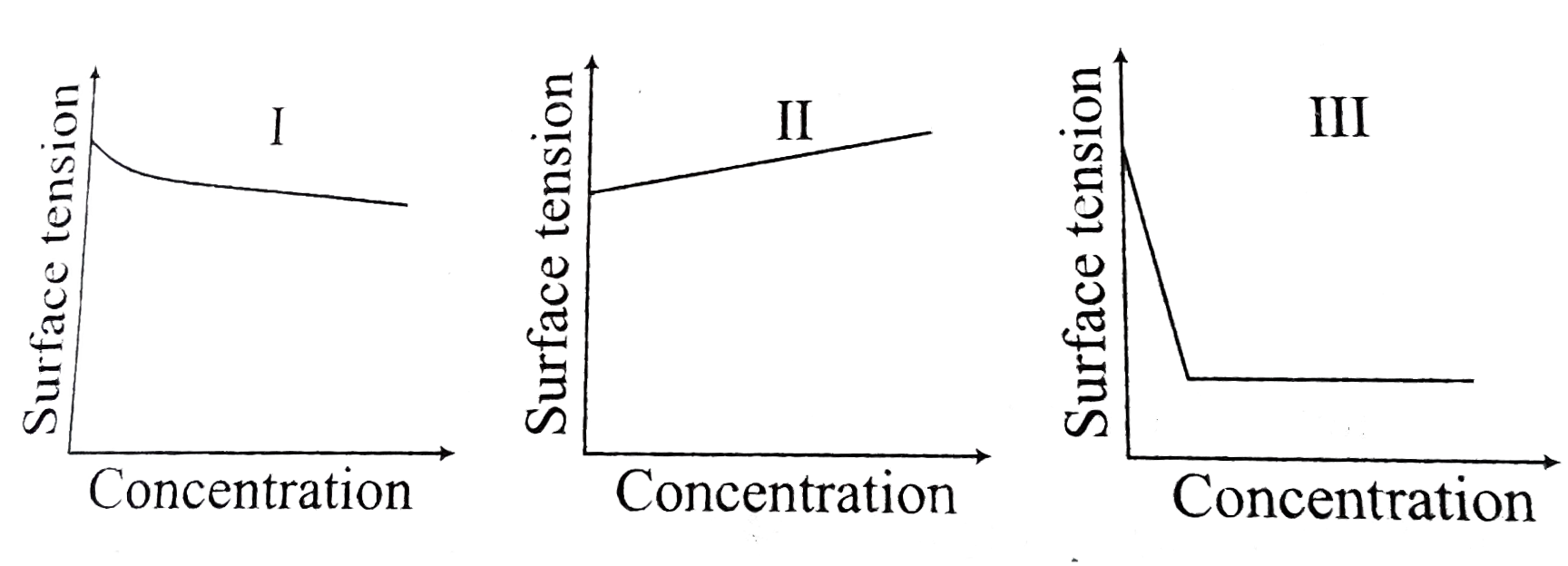
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- The correct statement(s) about surface properties is (are)
Text Solution
|