Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GASEOUS STATE -EXERCISE-3
- Determine final pressure after the valve is left opened for along time...
Text Solution
|
- One mole of NH(4) Cl ( s) is kept in an open container & then covered ...
Text Solution
|
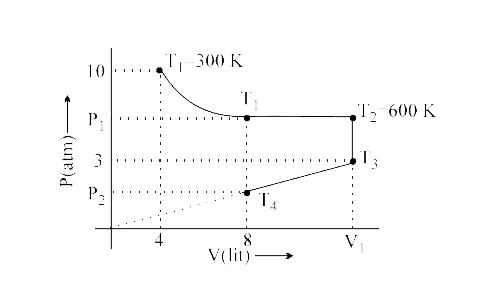
- Fixed mass of a gas is subjected to the changes as shown is diagram, c...
Text Solution
|
- A balloon containing 1 mole air at 1 atm initially is filled further w...
Text Solution
|
- One mole of an ideal gas is subjected to a process in which P =( 1)/( ...
Text Solution
|
- A compound exists in the gaseous phase both as monomer (A) and dimer (...
Text Solution
|
- During one of his adventure Chacha Chaudhary got trapped in an undergr...
Text Solution
|
- You are told to prepare a closed experimental environment ( a box) for...
Text Solution
|
- A closed vessel of known volume containing known amount of ideal gaseo...
Text Solution
|
- The apparatus shown consits of three temperature jacketed 1 litre bulb...
Text Solution
|
- Calcualte relative rate of effusion of SO(2) to CH(4) under given cond...
Text Solution
|
- A gas mixture contains equal number of molecules of N(2) and SF(6). So...
Text Solution
|
- Two gases NO and O(2) were introduced at the two ends of a 1m long tub...
Text Solution
|
- At 20^(@)C, two balloons of equal volume and porosity are filled to a ...
Text Solution
|
- Pure O(2) diffuses through an aperture in 224 second, whereas mixture ...
Text Solution
|
- Find he number of diffusion steps required to separated the isotopic m...
Text Solution
|
- The composition of the equilibrium mixture (Cl(2) 2Cl) , which is att...
Text Solution
|
- A mixture of CH(4) & O(2) is used as an optimal fuel if O(2) is presen...
Text Solution
|
- A 50 litre vessel is equally divided into three parts with the help of...
Text Solution
|
- Calculate the temperature values at which the molecules of the first t...
Text Solution
|