A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GASEOUS STATE -EXERCISE-4( LEVEL-II)
- The average velocity of gas molecules is 400 m/sec calculate its rms v...
Text Solution
|
- For one mole of gas the average kinetic energy is given as E.The U("rm...
Text Solution
|
- Ratio of rates of diffusion of He and CH(4) (under identical condition...
Text Solution
|
- Figure displays the plot of the compression factor Z verses pfor a few...
Text Solution
|
- Match gases under specified condition listed in Column I with their pr...
Text Solution
|
- A gas described by van der Waals equation
Text Solution
|
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- At 400 K, the root mean square (rms) speed of a gas X (molecular weigh...
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|
- To an evacuated vessel with movable piston under external pressure of ...
Text Solution
|
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- The atomic masses of He and Ne are 4 and 20 amu respectively . The va...
Text Solution
|
- A fixed mass m of a gas is subjected to transformation of state: K to ...
Text Solution
|
- A fixed mass 'm' of a gas is subjected to transformation of states fro...
Text Solution
|
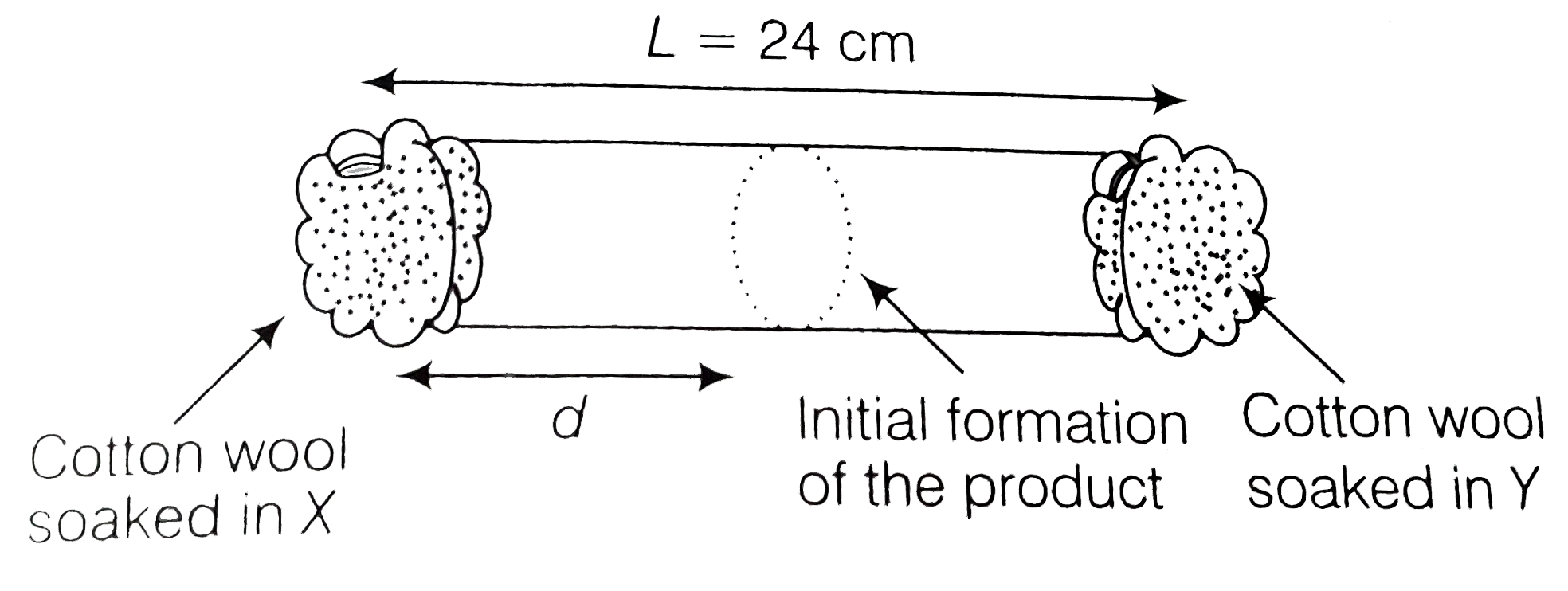
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh...
Text Solution
|
- The diffusion coefficient of an ideal gas is proportional to its mean ...
Text Solution
|
- A closed tank has two compartments A and B, both filled with oxygen (a...
Text Solution
|