Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-THERMOCHEMISTRY -Exercise - 4 Level-II
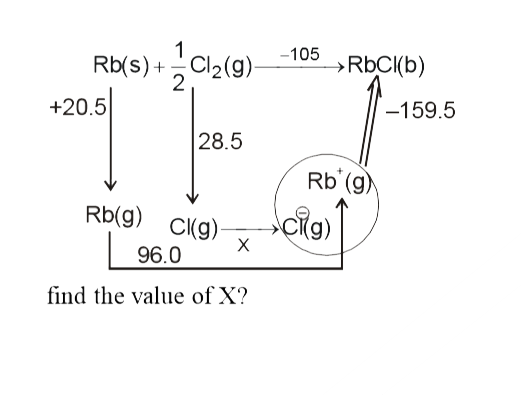
- The born-Haber cycle for formation of rubidium chloride (RbCl) is give...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|
- The bond energy (in kcal mol^(-1)) of a C -c single bond is approximat...
Text Solution
|
- The species which by definition has zero standard molar enthalpy of fo...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- The standard enthalpies fo formation of CO(2) (g), H(2) O (1), and glu...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|