Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
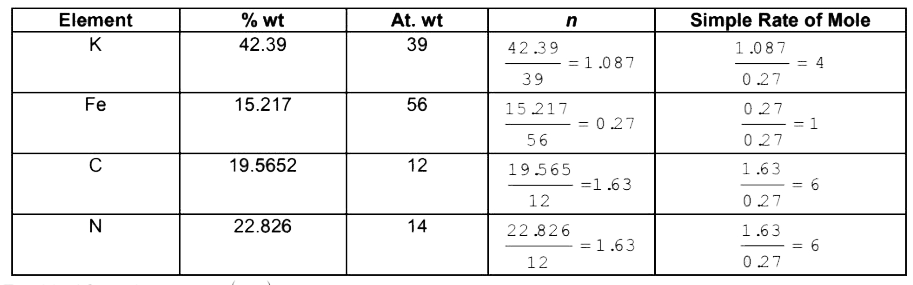
- A compound contains 42.3913 % K,15.2173 % Fe,19.5652 % C and 22.8260 %...
Text Solution
|
- A compound contains 42.3913 % K,15.2173 % Fe,19.5652 % C and 22.8260 %...
Text Solution
|
- A crystalline compound when heated become anhydrous by losing 51.2 % o...
Text Solution
|
- Compound 'A' is found to contain 36.5 % Na,25.4 % S and 38.1 % O . Its...
Text Solution
|
- An organic compound contains 49.3 % carbon,6.84 % hydrogen and its vap...
Text Solution
|
- A compound contains 38. 8 % C, 16 % H, 42 5 % N. The formula of comp...
Text Solution
|
- A crystalline compound when heated became anhydrous by losing 51.2 % o...
Text Solution
|
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|
- किसी यौगिक में 50% कार्बन, 50% ऑक्सीजन तथा इसका अणुभार लगभग 290 है। इस...
Text Solution
|