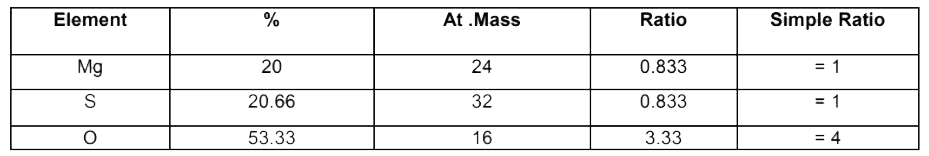Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A crystalline compound when heated became anhydrous by losing 51.2 % o...
Text Solution
|
- A compound contains 42.3913 % K,15.2173 % Fe,19.5652 % C and 22.8260 %...
Text Solution
|
- A crystalline compound when heated become anhydrous by losing 51.2 % o...
Text Solution
|
- An organic compound on analysis gave the following percentage composit...
Text Solution
|
- A crystalline salt on being rendered anhydrous loses 45.6 % of its wei...
Text Solution
|
- Compound 'A' is found to contain 36.5 % Na,25.4 % S and 38.1 % O . Its...
Text Solution
|
- An organic compound on analysis gave C=39.9 % ,H= 6.7 % and O =53.4 % ...
Text Solution
|
- A crystalline salt when heated beocmes anhydrous and loses 51.2% of it...
Text Solution
|
- A crystalline compound when heated became anhydrous by losing 51.2 % o...
Text Solution
|