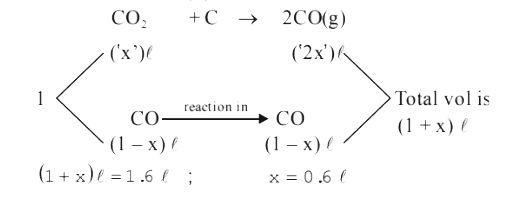Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1 L of a mixture of CO and CO(2), when passed through red hot tube con...
Text Solution
|
- One litre of mixture of CO and CO(2) is passed through red hot charcoa...
Text Solution
|
- (a). Take 1 L of a mixture of CO and CO(2) Pass this mixture through a...
Text Solution
|
- One litre of a mixture of CO and CO2 is passed through red-hot charcoa...
Text Solution
|
- When burnt in air, a 12.0 g mixture of carbon and sulphur yields a mix...
Text Solution
|
- A 100 ml mixture of CO and CO2 is passed through a tube containing red...
Text Solution
|
- 1 L of a mixture of CO and CO(2), when passed through red hot tube con...
Text Solution
|
- One litre of CO(2) is passed through red hot coke, The volume becomes ...
Text Solution
|
- When burnt in air, 14.0g mixture of carbon and sulphur gives a mixture...
Text Solution
|