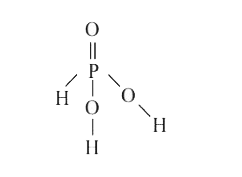
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The normality of 0.3 M phosphorous acid (H(3) PO(3)) is
Text Solution
|
- The normality of 0.3 M phosphorous acid (H(3) PO(3)) is
Text Solution
|
- The normality of 0.3M phosphorous acid H(3)PO(3) is:
Text Solution
|
- The normally of 0.3 M phosphorus acid (H(3) PO(3)) is
Text Solution
|
- The normality of 0.3 M phosphorous acid (H(3)PO(3)) is :
Text Solution
|
- Phosphoric acid H(3)PO(4) can not be neutralised to :
Text Solution
|
- The normality of 0.3 M phosphorus acid (H(3)PO(3)) solution is :
Text Solution
|
- What is the normality of 0.3 M H(3)PO(4) solution ?
Text Solution
|
- 0.3 M फॉस्फोरस अम्ल (H(3)PO(3)) की नॉर्मलता है-
Text Solution
|