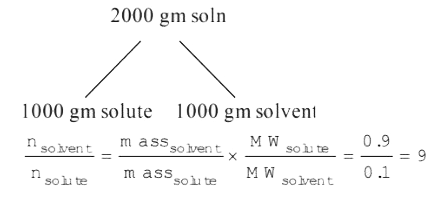
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The mole fraction of a solute in a solutions is 0.1. At 298K molarity ...
Text Solution
|
- The expression relating mole fraction of solute (chi(2)) and molarity ...
Text Solution
|
- The molality of a solute in a solvent (molar mass, 20g mol^(-1)) is 0....
Text Solution
|
- If m= molality of solution (mol Kg^(-1)) chi(solute)= mole fractio...
Text Solution
|
- The mole fraction of a solute in a solutions is 0.1. At 298K molarity ...
Text Solution
|
- A solution is made by dissolving CaBr(2) in water (solvent) such that ...
Text Solution
|
- The mole fraction of a solute in a solutions is 0.1. At 298K molarity ...
Text Solution
|
- If the density of the solvent is 2.5 kg dm^(-3) . The 2 molal solution...
Text Solution
|
- The mole fraction of a solute in a solution is 0.1. At 298 K, molarity...
Text Solution
|