A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
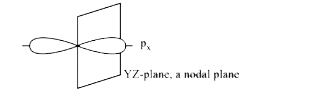
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The number of nodal planes in a px orbital is.
Text Solution
|
- In case of p(z) orbital, theis a nodal plane.
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The number of nodal planes in p(x) -obital is:
Text Solution
|
- p(x) कक्षक में नोडल तलों कि संख्या हैं :
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- Number of nodal planes for f-orbital are
Text Solution
|
- p(x)- कक्षक में नोडल तलों की संख्या है
Text Solution
|