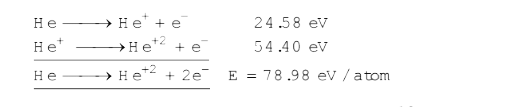Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The first and second ionization potentials of helium atoms are 24.58 e...
Text Solution
|
- The ionisation potential of hydrogen is 13.60 eV. Calculate the energy...
Text Solution
|
- The ionization potential for hydrogen atom is 13.6 eV , the ionization...
Text Solution
|
- Assertion Energy E(1) is required to remove first electron from helium...
Text Solution
|
- If first orbit energy of He^(+) is -54.4 eV, then the second orbit ene...
Text Solution
|
- The ionization potential of hydrogen is 13.6 0 eV. Calculate the energ...
Text Solution
|
- The first and second ionization potentials of helium atoms are 24. 58 ...
Text Solution
|
- The ionization potential of hydrogen is 13.60 eV. Calculate the energy...
Text Solution
|
- The first and second ionization potentials of helium atoms are 24.58 e...
Text Solution
|