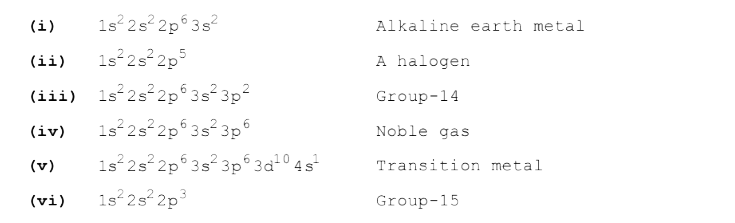Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The electronic configurations of a few elements are listed: (i) 1s^(...
Text Solution
|
- Which of the following represent ground state configurations and whic...
Text Solution
|
- 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(5) is not the electron configuration...
Text Solution
|
- Name the elements that correspond to the given electronic configuratio...
Text Solution
|
- कुछ तत्वों के इलेक्ट्रॉनिक विन्यास नीचे दिये गये है। इन तत्वों को आवर्...
Text Solution
|
- Identify the atoms that have the following ground state electronic con...
Text Solution
|
- Consider the following list of electron configurations: (1) 1s^(2)2s...
Text Solution
|
- To which element do the following electronic configuration correspond ...
Text Solution
|
- Electronic configuration 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(1) Gives an...
Text Solution
|