A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
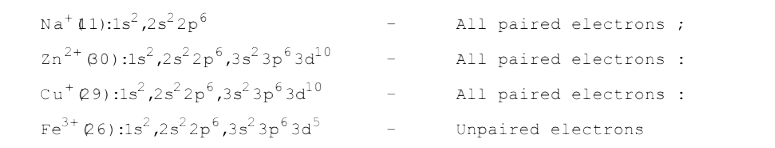
- The paramagnetic species among the following is- Na^(+), Zn^(2+), Cu^(...
Text Solution
|
- Among the following, the paramagnetic species is:
Text Solution
|
- The paramagnetic species among the following is- Na^(+), Zn^(2+), Cu^(...
Text Solution
|
- Increasing order of magnetic monent among the following species is Na^...
Text Solution
|
- The no. of paramagnetic species in the following . Na^(+),Cl^(-), Sn^(...
Text Solution
|
- Find the number of colourless species among the following: Sc^(3+),Ti^...
Text Solution
|
- Which of the following species is/are paramagnetic? Fe^(2+),Zn^(0),H...
Text Solution
|
- Which of the following species is/are paramagnetic Fe^(2+),Zn^(0),H...
Text Solution
|
- Which is most paramagnetic among Cu^(2+), Fe^(2+) and Cr^(3+) and why?
Text Solution
|