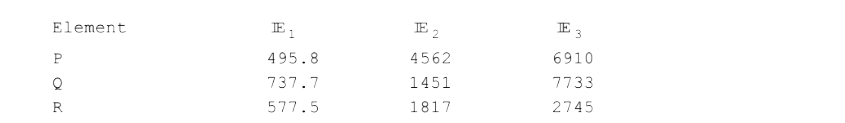A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- First three ionisation energies (in kJ/mol) of three representative el...
Text Solution
|
- Ionisation energies three hypothetical elements are given below (in kJ...
Text Solution
|
- Ionisation energies three hypothetical elements are given below (in kJ...
Text Solution
|
- The first three successive ionisation energies of an element X are 520...
Text Solution
|
- The first three successive ionisation energies of an element Y are 900...
Text Solution
|
- The first three successive ionisation energies of an element Z are 140...
Text Solution
|
- The first three successive ionisation energies of an element M are 800...
Text Solution
|
- First three ionisation energies (in kJ/mol) of three representative el...
Text Solution
|
- First three ionisation energies (in kJ/mol) of three representative el...
Text Solution
|