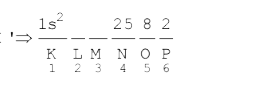Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- The total number of range of electrons in penultimate shell of d-block...
Text Solution
|
- Total number of electrons present in the penultimate shell of an eleme...
Text Solution
|
- An element has 13 electrons in its M shell and 1 electron in N shell i...
Text Solution
|
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- In the ground state, an element has 13 electrons in its M-shell. The e...
Text Solution
|
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- An element has 2 electrons in its K-shell. 8 electrons in L-shell, 13 ...
Text Solution
|