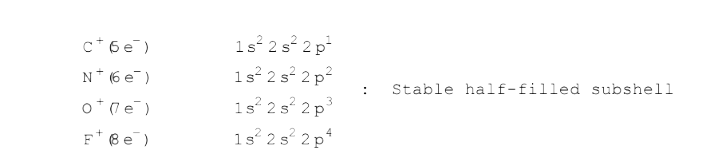A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct order of the second ionisation potential of carbon, nitrog...
Text Solution
|
- The correct order of the second ionisation potential of carbon, nitrog...
Text Solution
|
- The second ionisation potentials in electron volts of oxygen and fluor...
Text Solution
|
- The correct increasing order of the atomic radii of the elements oxyge...
Text Solution
|
- The correct order of the second ionisation potential of carbon, nitrog...
Text Solution
|
- The correct order of second ionisation potential of carbon, nitrogen, ...
Text Solution
|
- The correct increasing order of the atomic radii of the elements oxyge...
Text Solution
|
- The correct order of first ionisation enthalpy of carbon,nitrogen,oxyg...
Text Solution
|
- Second ionisation potential of oxygen is
Text Solution
|