Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Why the first ionisation energy of carbon atom is greater than that of...
Text Solution
|
- Why the first ionisation energy of carbon atom is greater than that of...
Text Solution
|
- The first ionisation energy of magnesium is greater than that of sodiu...
Text Solution
|
- Assertion: Ionisation energy of atomic hydrogen is greater than atomic...
Text Solution
|
- The first ionisation energy of first atom is greater than that of seco...
Text Solution
|
- The first ionization enthalpy of carbon atom is greater than that of b...
Text Solution
|
- The first ionisation potential of carbon is but the second ionisation ...
Text Solution
|
- First ionisation potential of C- atom is greater than that of B atom ,...
Text Solution
|
- निम्न की व्याख्या कीजिए ''कार्बन परमाणु की प्रथम आयनन ऊर्जा बोरॉन प...
Text Solution
|
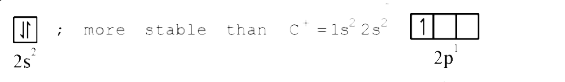 ionization trend is reversed due to stability of completely filled 2s-orbital of `B^(+)`
ionization trend is reversed due to stability of completely filled 2s-orbital of `B^(+)`