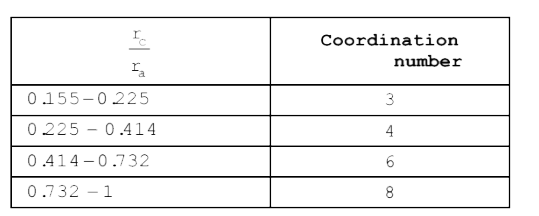
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The radius ratio (r(+)//r(-)) of an ionic solid (A^(+)B^(-)) is 0.69. ...
Text Solution
|
- Two ionic solids AB and CB crystallize in the same lattice. If r(A^(o+...
Text Solution
|
- Two ionic solid AB and CB crystallise in the same lattice. If r(A^(+))...
Text Solution
|
- In an ionic solid r((+))=1.6A and r((-))=1.864A. Use the radius ratio...
Text Solution
|
- The ionic radii for Na^(+) and Br^(-) ions a 1.012Å and 1.973Å respect...
Text Solution
|
- For tetrahedral coordination number, the radius ratio (r(c^(+)))/(r(a^...
Text Solution
|
- समन्वय संख्या छ: (अष्टफलकीय विन्यास) के लिए सीमांत त्रिज्या अनुपात ((r...
Text Solution
|
- आयनिक ठोसों में त्रिज्या अनुपात से क्या अभिप्राय है ? किसी आयनिक ठोस क...
Text Solution
|
- An ionic crystal has r(c)//r(a) radius of 0.542. Its coordination numb...
Text Solution
|