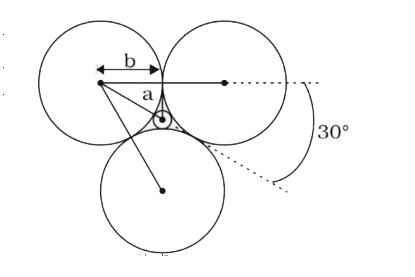
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The cation/anion radius ratio for a triangular arrangement of anions i...
Text Solution
|
- The range of radius ratio (cationic to anionic) for an octahedral arra...
Text Solution
|
- The radius ratio of the cation to the anion of an ionic compound is 0....
Text Solution
|
- Cation and Anions
Text Solution
|
- In which of the following the ratio of the sizes of cation to a...
Text Solution
|
- IF the radius ratio of cation to anion is in the range of 0.2...
Text Solution
|
- The range of radius ratio (cationic to anionic) for an octahedral arra...
Text Solution
|
- Which one of the following has minimum value of size of cation/anion r...
Text Solution
|
- The cation/anion radius ratio for a triangular arrangement of anions i...
Text Solution
|